Answers
Answer:
The individual substances in a mixture can be separated using different methods, depending on the type of mixture. These methods include filtration, evaporation, distillation and chromatography.
Explanation:
Related Questions
Please helpp asappp!!!!!!!!!!!!
water boils at 100*c. kayla measures the temperature of boiling water three times and receives the following results: 96.6*c, 96.8*c, and 96.5*c. which best describes her measurements?
1. they are more precise than accurate.
2. they are both precise and accurate.
3. they are more accurate than precise.
4. they are neither precise nor accurate.
Answers
water boils at 100*c. kayla measures the temperature of boiling water three times and receives the following results: 96.6*c, 96.8*c, and 96.5*c. 2. they are both precise and accurate best describes her measurements
Both accuracy and precision are methods to measure results. The extent of accuracy measures how near the results to the true or transfer is the transfer. Precision, on either hand, measures how similar two are. The accuracy of a measurement refers towards how close it is to the true values. Precision refers to how closely two measurements of the exact same item are. Precision is distinct from accuracy. You may also be precise while also being accurate. For example, if your measured values for a given material are close to the actual value on average, but the measured values are far apart, you have accuracy without precision.
learn more about accuracy here:
https://brainly.com/question/15276983
#SPJ4
I've tried looking it up but I can't find an answer. Please help with this question.

Answers
Answer:
Hydrocarbons
Explanation:
Answer:
Monomers
Explanation:
What is the product of the reaction of propanamide with methylmagnesium bromide (1 eq.)?
Answers
The reaction of propanamide with methylmagnesium bromide (1 eq.) yields a tertiary amide.
Reversible addition-elimination nucleophilic substitution reactions take place in this reaction mechanism, involving the bimolecular attack of the nucleophile (methylmagnesium bromide) and the displacement of the leaving group (bromide) from the substrate (propanamide).
This yields a carbocation intermediate, which is then attacked by water. The resulting alcohol undergoes dehydration through an acid-catalyzed cyclization to form an α,β-unsaturated intermediate. After protonation, the π-bond of the intermediate is eliminated and the tertiary amide is formed.
The overall stoichiometry of the reaction is 1:1, with one equivalent of propanamide and one equivalent of methylmagnesium bromide reacting to form the product tertiary amide. This reaction is useful in organic synthesis, as the tertiary amide can be hydrolyzed in acidic media to produce an amine, which can be used in further synthetically reactions.
know more about tertiary amide here
https://brainly.com/question/33301535#
#SPJ11
The total number of calcium atoms in the expression 3 cos 2 shown in the equation 3 CaCl 2 +2Na 3 PO 4 Ca(PO 4 ) 2 +6 NaCl is:

Answers
Answer:
\(C\)Explanation:
Here, we want to calculate the percentage composition of the compound formed when oxygen reacts with iron
We have the equation of reaction as follows:
\(4Fe_{(s)}\text{ + }3O_{2(g)}\text{ }\rightarrow\text{ }2Fe_2O_{3(s)}\)The compound formed is Fe2O3
Now, let us get its percentage composition
The molar mass of the compound is 160 g/mol
The atomic mass of iron is 56 amu
The atomic mass of oxygen is 16 amu
Now, let us get the percentage composition:
\(\begin{gathered} \text{Iron = }\frac{2\times56}{160}\text{ }\times\text{ 100 \% = }70\text{ \%} \\ \\ \text{Oxygen = }\frac{3\times16}{160}\text{ = 30\% } \end{gathered}\)The closest here is thus the option C
A balloon contains 2.0 L of air at 101.5 kPa. you squeeze the balloon to a volume of 0.5 L. what is the pressure of air inside the balloon?"
A. 13 kPa
B. 101 kPa
C. 406 kPa
D. 812 kPa
Answers
Answer:
C
Explanation:
view the Attached photo i added to see how i got that answer

What is the concentration of a solution containing 0.32 moles of NaCl in 3.4 liters?
Answers
Answer:
To get the molarity we need to divide the number of moles of NaCl by the volume of the solution. So, 0.32 moles NaCl divided by 3.4 L, and that gives 0.094 M NaCl.
what is the molality of a solution that contains 75.0 g of Cul dissolved in 1.50 kg of solvent
Answers
The molality of a solution that contains 75.0 g of Cul dissolved in 1.50 kg of solvent is 0.263 molal.
Molality is one of the important properties of solutions. It is used to express the concentration of a solute in a solution and mostly depends on the mass of the solvent. Molality is also sometimes referred to as molal concentration.
The number of moles of solute in a solution corresponding to 1 kg or 1000 g of solvent is known as molality. The definition of molarity, on the other hand, is based on a certain volume of solution.
Given,
Mass of solute = 75g
Mass of solvent = 1.5 kg
Molality = number of moles of solute / mass of solvent in kg
moles of solute = mass of solute / molar mass
= 75 / 190
= 0.394 moles of solute
Molality = 0.394 / 1.5
= 0.263 molal
Learn more about Molality, here:
https://brainly.com/question/26921570
#SPJ1
Draw the lewis structure for sio2 and indicate how many unshared pairs of electron are present on the silicon?
Answers
According to the given statement the structure for SIO2 is given below.
What is SIO2?Silica, commonly referred to as silicon dioxide, is a substance with the chemical formula SiO2. Two oxygen atoms and one silicon atom make up its composition. It is primarily found in sand.
Silicon Dioxide (SiO2) Lewis StructureSiO2's Lewis structure and CO's Lewis structure are the same. The sole distinction is that silicon is utilized in place of carbon.
The central atom is one of silicon, and two oxygen atoms are joined to it by a double bond. On the core atom of the SiO2 Lewis dot structure, there are no lone pairs.
Step 1 is to determine how many valence electrons are present in SiO2.
Step 2 is to locate the atom with the lowest electronegativity and place it in the center.
Step 3: Join each oxygen atom to the silicon atom with a single link.
Step 4: Arrange the remaining valence electrons, starting with the outermost atom.
Finish the center atom's octet in step five, and if possible, create a covalent link.
To know more about Lewis structure visit :
https://brainly.com/question/20300458
#SPJ4

Which action happens at the microscopic scale as the temperature of a substance decreases and it eventually freezes?
Answers
The speed of the particles rises with the temperature of the solid, liquid, or gas. The particles slow down with decreasing temperature.
A liquid can turn into a solid if it is cooled down far enough.
Why does a liquid become a solid when its temperature drops?The average kinetic energy of the molecules falls as a liquid cools.
The liquid eventually turns into a solid when the quantity of heat removed is sufficient to cause the molecules to be attracted to one another.
Freezing is the process of transitioning from a liquid to a solid.
It loses thermal energy when the liquid cools. Its constituent particles therefore decelerate down and converge.
learn more about kinetic energy refer
https://brainly.com/question/25959744
#SPJ13
What are all the Cells in a Plant?
Answers
Parenchyma cells.
Collenchyma cells.
Sclerenchyma cells.
Xylem cells.
Phloem cells.
Meristematic cells.
Epidermal cells.
Have a luvely day!
student carries out a titration to determine the concentration of a solution of
nitric acid. She titrates the solution of nitric acid against a standard solution
of sodium hydroxide with a known concentration of 0.0998 mol/dm². She
finds that 21.80 cm of the nitric acid solution is needed to exactly neutralise
25.0 cm of the sodium hydroxide solution.
Calculate the concentration of the nitric acid solution. Give your answer to
three significant figures.
The equation for the neutralisation reaction is
HNO3 + NaOH → NaNO3 + H2O
Answers
The concentration of the Nitric acid solution : 0.114 M
Further explanationTitration is a procedure for determining the concentration of a solution (analyte) by reacting with another solution whose known concentration (usually a standard solution) is called the titrant. Determination of the endpoint/equivalence point of the reaction can use indicators according to the appropriate pH range
Titrations can be acid-base titration, depositional titration, and redox titration. An acid-base titration is the principle of neutralization of acids and bases
Reaction
HNO₃ + NaOH → NaNO₃ + H₂O
Concentration a standard solution of sodium hydroxide : 0.0998 mol/dm³ , and the volume = 25 cm³
moles NaOH=
\(\tt mol=M\times V\\\\mol=0.0998\times 25\\\\mol=2.495~mlmoles\)
From the equation, mol ratio HNO₃ : NaOH = 1 : 1, so mol HNO₃ = mol NaOH=2.495 mlmoles
The volume of HNO₃ = 21.8 cm³, so the concentration :
\(\tt M=\dfrac{n}{V}\\\\M=\dfrac{2.495}{21.8}\\\\M=0.114\)
The concentration of the nitric acid solution is 0.11445 mol/dm³
From the question,
We are to calculate the concentration of the nitric acid solution
The given balanced chemical equation for the reaction is
HNO₃ + NaOH → NaNO₃ + H₂O
This means 1 mole of HNO₃ is needed to completely neutralize 1 mole of NaOH
Using the formula
\(\frac{C_{A}V_{A} }{C_{B}V_{B}} = \frac{n_{A}}{n_{B}}\)
Where \(C_{A}\) is the concentration of acid
\(C_{B}\) is the concentration of base
\(V_{A}\) is the volume of acid
\(V_{B}\) is the volume of base
\(n_{A}\) is the mole ratio of acid
\(n_{B}\) is the mole ratio of base
From the given information
\(C_{B}= 0.0998\ mol/dm^{3}\)
\(V_{A} = 21.80 \ cm^{3}\)
\(V_{B} = 25.0 \ cm^{3}\)
From the balanced chemical equation
\(n_{A} = 1\)
\(n_{B} =1\)
Putting the parameters into the formula, we get
\(\frac{C_{A} \times 21.80 }{0.0998 \times 25.0} = \frac{1}{1}\)
Then,
\(C_{A} \times 21.80=0.0998 \times 25.0\)
∴ \(C_{A}=\frac{0.0998 \times 25.0}{21.80}\)
\(C_{A} =\frac{2.495}{21.80}\)
\(C_{A} = 0.11445 \ mol/dm^{3}\)
Hence, the concentration of the nitric acid solution is 0.11445 mol/dm³
Learn more here: https://brainly.com/question/14014457
so no one go help me wit my chemistry huh
Answers
Answer:
depends...
on how difficult it is
Calculate the atomic mass of nitrogen, given that the percent abundance of nitrogen-14 and nitrogen-15 are 99.4% and 0.600% respectively.
Answers
Answer:
idrk im sorry and iknow this didnt help bt im sorry
Explanation:
why did the height (and volume) change in the test solution? what is the basis for the increase in the volume in the test solution?
Answers
The reason for the change in height (and volume) in the test solution is due to the introduction of a solute into the solvent, which causes the solution's volume to increase.
The basis for the increase in the volume of the test solution is the intermolecular forces between the solute and solvent molecules.
When a solute is added to a solvent, it interacts with the solvent molecules through various intermolecular forces, such as hydrogen bonding, dipole-dipole interactions, and London dispersion forces. These interactions cause the solute and solvent molecules to become more tightly packed, which leads to an increase in the volume of the solution.
The increase in volume is also due to the fact that the solute molecules take up space within the solvent, which leads to an overall increase in the volume of the solution. This increase in volume can be measured using techniques such as titration or dilution, which are commonly used in analytical chemistry.
To learn more about solvent, click here:
https://brainly.com/question/30885015
#SPJ11
A diagonal boundary seems to divide at least layer f and layer b into two sections. predict the event that formed this boundary, and predict whether the event occurred before or after the formation of the layers around it. explain your reasoning.
Answers
The equator is a hypothetical line passing through the center of the planet at latitude 0°. The Northern Hemisphere and the Southern Hemisphere are the two equal halves of the earth that are separated by the equator.
Greater gravitational force is exerted on the mass of denser objects and materials. This explains why materials with varying densities stack together
Which activities would take place at a transform boundary?At a transform plate border, the grinding motion between the plates causes shallow earthquakes, significant lateral rock displacement, and a wide zone of crustal deformation. Along the San Andreas Fault in western California, a landscape of this kind may be seen more spectacularly than anywhere else on Earth.
What are the core's two split components?The inner core and the outer core, which encircle the mantle, are the two layers that make up the core. The Bullen discontinuity is the line dividing these two regions. Radius Core The 2,200 kilometers (1,367 miles) thick outer core is primarily made of liquid nickel and iron.
What layers of the Earth is represented by layer 1 and layer 2?
Composition-based Layers
The following layers are visible in a cross section of the Earth: First, there is the crust, followed by the mantle, the outer core, the inner core, the lithosphere, the asthenosphere, the outer core, and the inner core. Based on composition, the earth's core, mantle, and crust are divided: Less than 1% of the mass of Earth is made up of the crust.
To know more about Earth's layers formation, visit:https://brainly.com/question/19566796
#SPJ4
What might happen if the mouse was removed from this
food web? Please be specific and mention ALL organisms
affected.
I’ll give u brainliest hurry
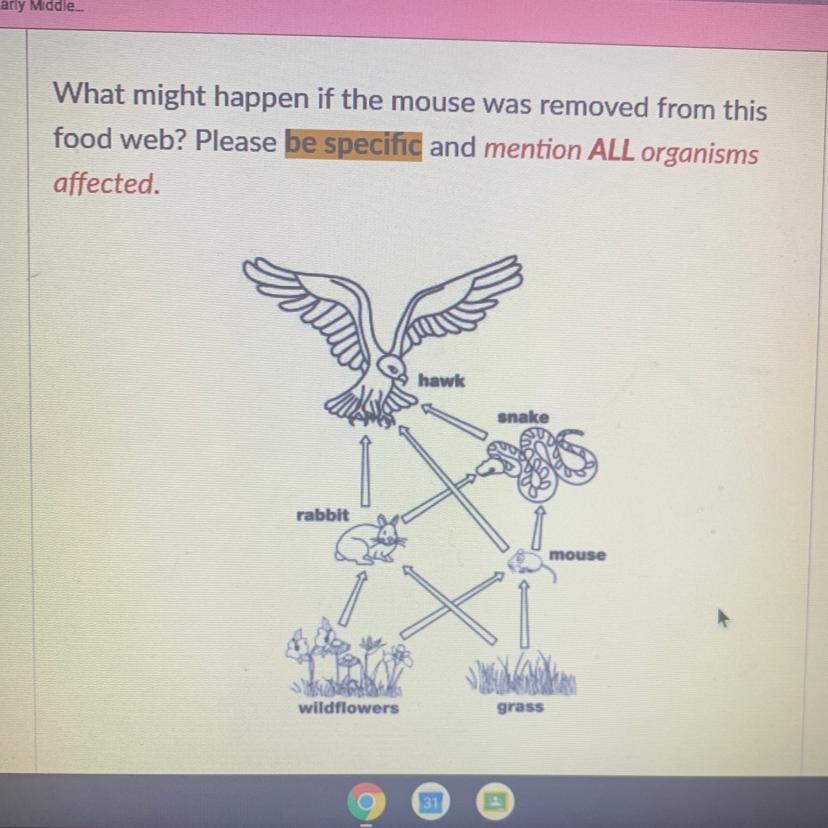
Answers
Answer:
They would most likely starve and die UNLESS they could move to another habitat or tend to eating other sources of food. All the other animals in the food web would die too, because their food supplies would have gone. The populations of the consumers would fall as the population of the producer fell.
When a organism is removed, the organism who eats or hunt them will decrease some because they lost one of the food source even though they still have other food sources. This new organism will brake the balance of the ecosystem so their food sources will decrease by having too many predators that hunt and eat them.
In food chain's if one source from it is ever removed it would mess up the entire chain causing issue
what is the molarity of a nitric acid solution HNO3 if 20 ml of the solution is needed to exactly neutralize 10 ml of a 1.67 M NaOH solution?
Answers
Answer: 0.84 M
\(M_{A}V_{A}=M_{B}V_{B}\\(20)M_{B}=10(1.67)\\M_{B}=\frac{10(1.67)}{20} \approx \boxed{0.84 \text{ M}}\)

what is the difference between simple sugars and complex carbohydrates
Answers
Simple sugars are single sugar molecules that are quickly digested and absorbed, while complex carbohydrates are polysaccharides that take longer to break down, providing sustained energy and additional nutrients.
Simple sugars, also known as monosaccharides or simple carbohydrates, are single sugar molecules that are easily digested and rapidly absorbed into the bloodstream. They include glucose, fructose, and galactose. Simple sugars are naturally found in fruits, honey, and milk, and they are also added to many processed foods and beverages as sweeteners. Due to their molecular structure, simple sugars provide quick bursts of energy but lack substantial nutritional value.
On the other hand, complex carbohydrates are polysaccharides composed of multiple sugar molecules linked together. They are found in foods such as whole grains, legumes, vegetables, and starchy foods like potatoes and corn. Complex carbohydrates take longer to break down during digestion due to their complex structure, resulting in a slower and more sustained release of glucose into the bloodstream. This slower digestion process helps maintain stable blood sugar levels, provides sustained energy, and promotes a feeling of fullness.
The key difference between simple sugars and complex carbohydrates lies in their molecular structure and how they affect the body. Simple sugars are quickly absorbed and can lead to rapid blood sugar spikes, which may contribute to energy crashes and cravings. Complex carbohydrates, with their longer digestion time, provide a more gradual release of energy, promote satiety, and offer additional nutrients, such as fiber, vitamins, and minerals. Incorporating a balanced mix of both simple and complex carbohydrates into the diet is important for overall health and energy management.
Know more about Complex Carbohydrates here:
https://brainly.com/question/28777139
#SPJ11
If 3.00 mL of 0.0250 M CuSO4 is diluted to 25.0 mL with pure water, what is the molarity of copper(II) sulfate in the diluted solution
Answers
Answer:
0.00268 M
Explanation:
To find the new molarity, you need to (1) find the moles of CuSO₄ (via the molarity equation using the beginning molarity and volume) and then (2) find the new molarity (using the moles and combined volume). Your final answer should have 3 sig figs to match the given values.
Step 1:
3.00 mL / 1,000 = 0.00300 L
Molarity = moles / volume (L)
0.0250 M = moles / 0.00300 L
(0.0250 M) x (0.00300 L) = moles
7.50 x 10⁻⁵ = moles
Step 2:
25.0 mL / 1,000 = 0.0250 L
0.0250 L + 0.00300 L = 0.0280 L
Molarity = moles / volume (L)
Molarity = (7.50 x 10⁻⁵ moles) / (0.0280 L)
Molarity = 0.00268 M
Help with this please it’s due at 11:59pm tonight

Answers
The molecular structure of the covalent molecules, as well as the electron dot diagrams of hydrogen, ammonia, hydrochloric acid, water, and methane, are found in the attachment.
What are covalent molecules?Covalent molecules are molecules that are formed by covalent bonds between two or more atoms of the same or different elements.
The inorganic elements hydrogen, fluorine, chlorine, water, and ammonia as well as all organic compounds are examples of molecules with covalent connections.
A covalent bond is formed when two atoms exchange one or more pairs of electrons. The two atomic nuclei are concurrently drawing these electrons to them. When the difference between the electronegativities of two atoms is too tiny for an electron transfer to take place to create ions, a covalent bond is formed.
In electron dot diagrams, the valence electrons of an atom are represented by dots that are positioned all around the symbol of the element.
Learn more about covalent molecules at: https://brainly.com/question/3447218
#SPJ1





Calcite (the main mineral in limestone) is made of calcium carbonate (caco3). dolomite, a related mineral, is made of magnesium carbonate (mgco3). what happens if a geologist drips a small amount of vinegar (acetic acid) onto a sample of dolomite? there is no way to predict what will happen. fizzing will occur because carbon dioxide is produced. no reaction will occur because dolomite contains no calcium.
Answers
If a geologist drips a small amount of vinegar (acetic acid) onto a sample of dolomite, fizzing will occur because carbon dioxide is produced.
Why Carbon dioxide show effervescence?Effervescence observes whenever any gas tried to escape out from an aqueous solution, that's why CO₂ show effervescence.
Reaction between dolomite and acetic acid will be represented as:
MgCO₃ + 2CH₃COOH → CO₂ + H₂O + Mg(CH₃COO)₂
From the above reaction it is clear that carbon dixide gas is produced by showing the effervescence and fizzing behavior.
Hence, option (2) is correct.
To know more about dolomite, visit the below link:
https://brainly.com/question/4945288
Answer:
B
Explanation:
on edge2022
can there be a negative number answer in a combined gas law problem
Answers
what occurs when a soluble mineral receives water through precipitation, causing it to weaken?
Answers
When a soluble mineral receives water through precipitation, it can weaken and dissolve due to the chemical reactions that occur between the mineral and the water.
When a soluble mineral receives water through precipitation, it can cause it to weaken and dissolve. Precipitation is a natural process where water vapor in the atmosphere condenses into liquid form and falls to the ground as rain, snow, sleet, or hail. When this precipitation comes into contact with soluble minerals such as salt, calcium, or magnesium, it can dissolve and weaken the mineral's structure.
The amount of precipitation and the solubility of the mineral will determine the extent of the weakening process. Some minerals are more resistant to dissolution than others, and certain types of precipitation like acid rain can accelerate the process of dissolution. The weakening of minerals through precipitation can have significant impacts on the environment, including soil erosion, water quality degradation, and damage to infrastructure.
In summary, when a soluble mineral receives water through precipitation, it can weaken and dissolve due to the chemical reactions that occur between the mineral and the water. This can have significant environmental and infrastructural impacts and is an important consideration in many fields of study.
To know more about precipitation visit: https://brainly.com/question/31198360
#SPJ11
3-less than or equal to -5x graph in slope intercept form question #9

Answers
9. The slope for the given equation 3- y ≤ -5x is 5 and intercept is 3.
What is meant by slope intercept method?Slope-intercept form (y=mx+b) of linear equations highlights the slope (m) and y-intercept (b) of line. The slope intercept formula y = mx + b is used when you know the slope of line that is to to be examined and point given is also the y intercept (0, b). In this formula, b represents y value of the y intercept point.
The slope intercept for of linear equation is: y = mx +b
m is slope and b is y intercept.
So, y = 5x + 3
Now m = 5 ; so the slope is 5
b = 3 ; hence y-intercept is 3
To know more about slope intercept method, refer
https://brainly.com/question/22057368
#SPJ1
In the kinetic molecular theory of gas behavior, the assumption is made that gas molecules:.
Answers
Answer:
In the kinetic molecular theory of gas behavior; the assumption is made that gas molecules move with a kinetic energy equal to their centigrade temperature_ move rapidly in random directions: are close together in their container which exerts pressure_ are attracted to each other by strong forces.
Explanation:
Water can exist in all three states of matter.
Describe the arrangement and movement of particles in each of the three states of matter. Explain what happens to the particles in a liquid during boiling.
I'll give someone brainly and thanks if they answered the question correctly. 30 points!!
Answers
Answer:
The particles in a liquid move very quickly due to heat while it is boiling and gradually turns to gas
4. A 100.0 mL sample of 0.2516 M aqueous barium chloride was treated with an excess of sulfuric acid. The
resulting barium sulfate precipitate was filtered, dried, and found to have a mass of 4.9852 g. Compute the
percentage yield of barium sulfate in this process.
Answers
Can alternative sources lower inflation and possibly the price of gas and groceries?
Answers
Answer:
Explanation:
"No", Limiting how much companies can charge will distort markets, they argue, causing shortages and exacerbating supply chain problems while only temporarily reducing inflation.
An element is composed of one type of atom with its own unique properties. TRUE OR FALSE
Answers
Answer:
It's true
Hope it helps:-)
Answer:
true
Explanation:
5. Geologists think that the inner and outer cores of Earth consist of
iron and nickel.
Answers
Answer:
is this true/false? if so its true.
Explanation:
