Answers
Instructions on balancing chemical equations:
Enter an equation of a chemical reaction and click 'Balance'. The answer will appear below
Always use the upper case for the first character in the element name and the lower case for the second character. Examples: Fe, Au, Co, Br, C, O, N, F. Compare: Co - cobalt and CO - carbon monoxide
To enter an electron into a chemical equation use {-} or e
To enter an ion specify charge after the compound in curly brackets: {+3} or {3+} or {3}.
Example: Fe{3+} + I{-} = Fe{2+} + I2
Substitute immutable groups in chemical compounds to avoid ambiguity.
For instance equation C6H5C2H5 + O2 = C6H5OH + CO2 + H2O will not be balanced,
but PhC2H5 + O2 = PhOH + CO2 + H2O will
Compound states [like (s) (aq) or (g)] are not required.
If you do not know what products are enter reagents only and click 'Balance'. In many cases a complete equation will be suggested.
Reaction stoichiometry could be computed for a balanced equation. Enter either the number of moles or weight for one of the compounds to compute the rest.
Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents
Related Questions
The cultures of prehistoric humans are known mostly through the excavation of stone tools and other relatively imperishable artifacts. The early tool making traditions are often referred to as being paleolithic (literally "Old Stone Age). The Oldowan and Acheulian tool traditions of the first humans were the simplest applied research basic research Scientihe thought O philosophies technologies
Answers
The cultures of prehistoric humans are primarily known through the excavation of stone tools and other durable artifacts, such as the Oldowan and Acheulian tool traditions.
Stone tools and imperishable artifacts serve as key archaeological evidence for understanding prehistoric cultures. Through meticulous excavation and analysis, archaeologists have been able to piece together the lifestyles, technological advancements, and social behaviors of early human societies. The term "paleolithic" refers to the Old Stone Age, a time when humans relied on stone tools as their primary implements.
The Oldowan tool tradition is considered the earliest stone tool industry, dating back around 2.6 million years ago. It is characterized by simple tools, such as choppers and scrapers, which were crafted by flaking off pieces from larger stones. These tools were primarily used for basic activities like butchering and processing animal carcasses.
Later, the Acheulian tool tradition emerged around 1.76 million years ago, representing an advancement in stone tool technology. Acheulian tools, such as handaxes and cleavers, were more refined and standardized, showcasing an increased level of sophistication in tool-making techniques. These tools served a wide range of purposes, including hunting, woodworking, and shaping raw materials.
By studying the Oldowan and Acheulian tool traditions, researchers gain valuable insights into the cognitive abilities, cultural development, and technological progress of early humans. The examination of these artifacts provides evidence of their adaptability, problem-solving skills, and the gradual refinement of their tool-making techniques over time.
Learn more about prehistoric humans
brainly.com/question/28301954
#SPJ11
if a pork roast must absorb 1700 kj to fully cook, and if only 12% of the heat produced by the barbeque is actually absorbed by the roast, what mass of co2 is emitted into the atmosphere during the grilling of the pork roast?express your answer using two significant figures.
Answers
Approximately 280.72 grams of CO2 are emitted into the atmosphere during the grilling of the pork roast.
The energy absorbed by the roast and the energy efficiency of the barbecue.
Given:
Energy absorbed by the pork roast = 1700 kJ
Energy efficiency of the barbecue = 12% = 0.12
Since only 12% of the heat produced by the barbecue is absorbed by the roast, we can calculate the total heat produced by the barbecue using the equation:
Total heat produced = Energy absorbed / Energy efficiency
Total heat produced = 1700 kJ / 0.12
Total heat produced ≈ 14166.67 kJ
The combustion of propane, which is commonly used in barbecues, produces approximately 56 g of CO2 per mole of propane burned.
To calculate the mass of CO2 emitted, we need to convert the total heat produced to moles of propane and then determine the corresponding mass of CO2.
Calculate the moles of propane burned:
Moles of propane = Total heat produced / Heat of combustion of propane
The heat of combustion of propane is approximately 2220 kJ/mol.
Moles of propane = 14166.67 kJ / 2220 kJ/mol
Moles of propane ≈ 6.38 mol
Calculate the mass of CO2 emitted:
Mass of CO2 = Moles of propane × Molar mass of CO2
The molar mass of CO2 is approximately 44 g/mol.
Mass of CO2 = 6.38 mol × 44 g/mol
Mass of CO2 ≈ 280.72 g
Learn more about emitted here
https://brainly.com/question/13490538
#SPJ11
1. Describe the motion of molecules in a solid. Why is gold only present in the
solid state?
Answers
Gold is present in its solid state because the metallic bonding in gold (the strong electrostatic attraction between positive gold atoms and the delocalised electrons) is very strong and needs a lot of energy to break. Hence, gold has a high melting point and so at average temperatures on Earth is found in its solid state.
As the motion of the molecules in minimum due to strong forces of attraction between the solid molecules as in case of gold and hence it is only found in solid state.
What are forces of attraction?
Forces of attraction is a force by which atoms in a molecule combine. It is basically an attractive force in nature. It can act between an ion and an atom as well.It varies for different states of matter that is solids, liquids and gases.
The forces of attraction are maximum in solids as the molecules present in solid are tightly held while it is minimum in gases as the molecules are far apart . The forces of attraction in liquids is intermediate of solids and gases.
The physical properties such as melting point, boiling point, density are all dependent on forces of attraction which exists in the substances.
Learn more about forces of attraction,here:
https://brainly.com/question/2396709
#SPJ2
Which tatement decribe what happen to the kinetic energy of the particle in the atmophere a they come into contact with the meteoroid? A. The kinetic energy of the urrounding air particle i converted to electrical energy. B. The average kinetic energy of the urrounding air particle increae. C. The kinetic energy of the urrounding air particle i converted to potential energy. D. The average kinetic energy of the urrounding air particle decreae
Answers
The option B is the correct answer, The average kinetic energy of the surrounding air particle increase.
An object's kinetic energy is the energy it has as a result of its motion. It is defined as the amount of work required to accelerate a given mass body from rest to a given velocity. Unless the body's speed changes, the kinetic energy gained during acceleration is retained. Kinetic energy is the energy that a moving object has.. If we want to accelerate an object, we must apply force to it. Using force necessitates exerting effort. When the work is finished, energy is transferred to the object, and it moves at a new constant speed.
To learn more about kinetic energy click here
brainly.com/question/26472013
#SPJ4
Complete Question
Which statement describe what happen to the kinetic energy of the particle in the atmosphere a they come into contact with the meteoroid? A. The kinetic energy of the surrounding air particle is converted to electrical energy. B. The average kinetic energy of the surrounding air particle increase. C. The kinetic energy of the surrounding air particle is converted to potential energy. D. The average kinetic energy of the surrounding air particle decrease
How many molecules are represented in the formula 2CaCO3?
Answers
I believe it would be 2, if CaCO3 is your molecule you will have two because of the number in front :)
The energy necessary for photosynthesis to take place is provided by
Question 3 options:
The sunlight
The oxygen in the air
The electricity in our homes
The heat in the soil
Answers
Answer:
The Sunlight
Oxegyn
Heat in soi
Explanation:
Answer:
sun, air, and heat.
Explanation:
hope this helps!
a student obtained a wet burette from the cart but failed to rinse it with a small amount of the base before starting a titration. will more or less titrant (base) be required to neutralize the acid?
Answers
The student failed to rinse a wet burette with a small amount of base before starting a titration. The student would need more titrant (base) to neutralize the acid than if they had rinsed the burette before starting the titration.
The reason for this is that when a wet burette is not rinsed with the titrant, the remaining water in the burette can dilute the titrant, thereby decreasing its concentration. If the titrant is diluted, more of it would be required to neutralize the same amount of acid. This would result in a titration that requires more titrant (base) to neutralize the acid than if the burette had been properly rinsed with the base.
In other words, not rinsing the burette with a small amount of base can affect the accuracy of the titration results. It is, therefore, important to properly rinse the burette with the titrant before starting a titration to avoid diluting the titrant and ensure accurate titration results.
In conclusion, more titrant (base) would be required to neutralize the acid if a wet burette is not rinsed with a small amount of base before starting a titration. It is essential to rinse the burette before starting a titration to avoid dilution of the titrant and ensure accurate titration results.
for more such question on titration
https://brainly.com/question/186765
#SPJ11
please answer quick please, im have a test

Answers
Complete the Lewis structures for diazine (C5H4N2) showing the two most common resonance forms. Draw the Lewis structures with the formal charges minimized.
Answers
Answer:
See explanation and image attached
Explanation:
Lewis structures dot structures that show the number of valence electron in a molecule. Sometimes, we show bonded electrons using a single line as in the image attached.
Two images are shown. They are the both resonance structures of diazene. The both structures only differ in the arrangement of electrons as we can see from the image.

_ _ _+Cl₂ ➡️ MgCl₂ Complete given Reaction
Answers
Answer:
MgExplanation:
Mg + Cl2 ----> MgCl2
plz follow me
Cocoa beans are subjected to three processes during the manufacture of chocolate: cleaning, roasting, and 'nibbing'. Bags of cocoa beans are first cleaned, then cleaned beans are roasted, then roasted
Answers
Beans are processed through 'nibbing'. During the nibbing process, the roasted cocoa beans are crushed and ground into a paste called cocoa mass or cocoa liquor.
This cocoa mass can then be further processed to separate the cocoa solids from the cocoa butter, which is the fat component of the cocoa bean. The separated cocoa solids and cocoa butter are used in the production of chocolate. Pure cocoa mass (cocoa paste) in solid or semi-solid form is known as chocolate liquor. It includes about equal amounts of cocoa butter and solid cocoa, much like the cocoa beans (nibs) from which it is made. It is made from fermented, dried, roasted, and separated from their skins cocoa beans. To make cocoa mass (cocoa paste), the beans are pulverised.
To know more about cocoa liquor
https://brainly.com/question/5047676
#SPJ11
Which of these is an extensive property of a substance?
O color
O hardness
O malleability
O volume
Answers
Answer:
volumeMassExplanation:
Extensive properties are ;
mass volume,Intensive properties are ;
density colour,Volume is an extensive property of a substance.
Which is an extensive property of a substance?An extensive property is a property that depends on the amount of matter in a sample. Mass and volume are examples of extensive properties. An intensive property is a property of matter that depends only on the type of matter in a sample and not on the amount.
Is density an extensive property?Density is an intensive property because there is a narrow range of densities across the samples. No matter what the initial mass was, densities were essentially the same. Since intensive properties do not depend on the amount of material, the data indicate that density is an intensive property of matter.
Learn more about the extensive property here https://brainly.com/question/17337720
#SPJ2
A person walks 2 miles in 30 minutes. Figure out how many feet per second they traveled.
Answers
Answer:
5.8667 ft/s
Explanation:
Is the rate of decay fastest at the beginning, middle, or end of the process?
Answers
Answer:
The beginning
Explanation:
In a nuclear reaction, the rate of decay is fastest at the beginning of the process.
What are nuclear reactions?There are two types of nuclear reactions which are nuclear fusion and nuclear fission .They involve the combination and disintegration of the element's nucleus respectively.
In nuclear fission, the nucleus of the atom is bombarded with electrons of low energy which splits the nucleus in to two parts .Large amount of energy is released in the process.It is used in nuclear power reactors as it produces large amount of energy.
In nuclear fusion,on the other hand, is a reaction which occurs when two or more atoms combine to form a heavy nucleus.Large amount of energy is released in the process which is greater than that of the energy which is released in nuclear fission process.
Learn more about nuclear reactions,here:
https://brainly.com/question/12649087
#SPJ2
How many grams of silver chromate will precipitate when 150. mL of 0.500 M silver nitrate are added to 100. mL of 0.400 M potassium chromate?
Answers
Approximately 7.98 grams of silver chromate will precipitate when 150 mL of 0.500 M silver nitrate is added to 100 mL of 0.400 M potassium chromate.
To determine the amount of silver chromate that will precipitate when 150 mL of 0.500 M silver nitrate is added to 100 mL of 0.400 M potassium chromate, we need to identify the limiting reagent and calculate the corresponding amount of silver chromate formed.
First, we can calculate the number of moles of silver nitrate and potassium chromate using their respective concentrations and volumes:
Moles of silver nitrate = concentration × volume = 0.500 M × 0.150 L = 0.075 mol
Moles of potassium chromate = concentration × volume = 0.400 M × 0.100 L = 0.040 mol
From the balanced chemical equation:
2 AgNO3 + K2CrO4 → Ag2CrO4 + 2 KNO3
We can see that the stoichiometric ratio between silver nitrate and silver chromate is 2:1. Therefore, the moles of silver chromate formed will be half the moles of silver nitrate used:
Moles of silver chromate formed = 0.075 mol / 2 = 0.0375 mol
Finally, we can calculate the mass of silver chromate using its molar mass:
Mass of silver chromate = moles × molar mass = 0.0375 mol × (2 × 107.87 g/mol) = 7.98 g
For more such questions on silver chromate
https://brainly.com/question/2854033
#SPJ8
calculate the energy processed by a single proton of the each following types electromagnetic radiation:
a.6.32 x 10^20 s-1
b. 9.50 x 10^13 hz
c.1.05 x10^16 s^-1
#G10 Chemistry
Answers
a. 4.2 x 10⁻¹³ J
b. 6.3 x 10⁻²⁰ J
c. 7 x 10⁻¹⁸ J
Further explanationRadiation energy is absorbed by photons
The energy in one photon can be formulated as
\(\large{\boxed{\bold{E\:=\:h\:.\:f}}}\)
Where
h = Planck's constant (6,626.10⁻³⁴ Js)
f = Frequency of electromagnetic waves
a.
\(\tt E=6.626.10^{-34}\times 6.32.10^{20}=4.1876.10^{-13}~J\)
b.
\(\tt E=6.626.10^{-34}\times 9.50.10^{13}=6.2947.10^{-20}~J\)
c.
\(\tt E=6.626.10^{-34}\times 1.05.10^{16}=6.9573.10^{-18}~J\)
describe one human activity, other than the burning of fossil fuels, that releases co2 into the atmosphere.
Answers
Cement production is a human activity that releases carbon dioxide into the atmosphere.
Cement production is a major contributor to greenhouse gas emissions, accounting for around 8% of global carbon dioxide emissions each year. Cement production is responsible for approximately 1.5 billion tonnes of carbon dioxide released each year, according to recent estimates. Cement production is an energy-intensive process that includes several steps that release carbon dioxide into the atmosphere. The main contributors to carbon dioxide release in the cement production process are the heating of limestone to produce calcium oxide (CaO) and the combustion of fossil fuels to provide heat for the kiln.
Learn more about carbon dioxide here.https://brainly.com/question/31200754
#SPJ11
is the following sentence true or false, in undisturb sedimentary rocks, the oldest layers are found on the bottom
Answers
Answer:
True.
Explanation:
this is because undisturbed sedimentary rocks forms in layers.
What is the acceleration acquired by an object that has a mass of 50kg and is pushed with a force of 20N.
Answers
If a 20 N force is applied to a 50 kg mass, then the acceleration acquired by the body is 0.4 m/s².
¿How to calculate the acceleration of a body?It is possible to know the acceleration of a body from Newton's second law, which states that the acceleration is defined as:
a = F/mWhere:
A = accelerationF = forceM = massTroubleshooting:We proceed to find the acceleration of the body, such that:
a = F/ma = 20N / 50kga = 0.4m/s²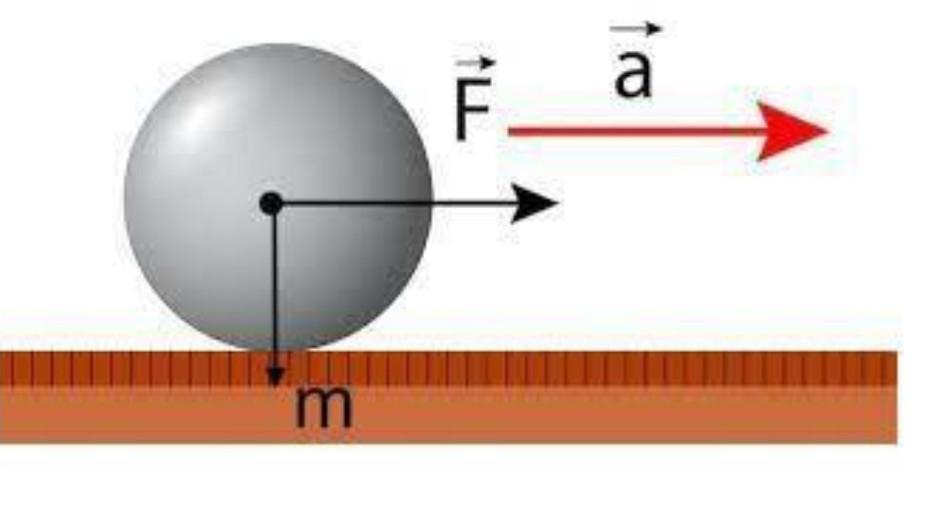
alculate the standard free-energy change at 25 ∘C for the following reaction:
Mg(s)+Fe2+(aq)→Mg2+(aq)+Fe(s)
Express your answer to three significant figures and include the appropriate units.
ΔG∘ =
Answers
The standard free-energy change at 25 ∘C for the given reaction, Mg(s)+Fe₂+(aq)→Mg₂+(aq)+Fe(s) can be calculated using the equation:ΔG∘ = ΣnΔGf∘ (products) − ΣnΔGf∘ (reactants)where ΔGf∘ is the standard free energy of formation of the compound, n is the stoichiometric coefficient of each compound in the reaction.
The values of ΔGf∘ for the given reaction are:
Mg₂+ (aq):−467.2 kJ/mol Fe(s): 0 kJ/mol Fe₂+(aq): −237.2 kJ/mol Mg(s): 0 kJ/molΔG∘ =
ΣnΔGf∘ (products) − ΣnΔGf∘ (reactants)ΔG∘ =
[ΔGf∘ (Mg₂+ (aq)) + ΔGf∘ (Fe(s))] - [ΔGf∘ (Mg(s)) + ΔGf∘ (Fe₂+(aq))]ΔG∘ =
[−467.2 kJ/mol + 0 kJ/mol] - [0 kJ/mol + (−237.2 kJ/mol)]ΔG∘ =
−230.0 kJ/mol At 25 ∘C, the standard free-energy change for the given reaction is −230.0 kJ/mol. The units of ΔG∘ are kilojoules per mole (kJ/mol). Hence, the answer is -230.0 kJ/mol.
To know more about stoichiometric coefficient refer here: https://brainly.com/question/32088573#
#SPJ11
5. The salts you tested were held together by ionic bonds. The compounds were made of two types of ions, positive and negative, that were attracted to each other because of their opposite charges. Some ionic bonds take more energy to break. Based on your investigation, which compounds have bonds that require the most energy to break?
Answers
Answer:
Lithium Iodide , Sodium Fluoride, Sodium Chloride, Sodium Bromide , Sodium Iodide, Potassium Fluoride and Potassium Chloride are the examples of ionic compounds.
Explanation:
Ionic compounds have bonds that require the most energy to break because of strong attractive force between them. According to scientists, about 700 to 4000 Kilo Joules energy is required to break bonds between ionic compounds whereas 800 to 1000 kilo joules energy is needed by the compounds having covalent triple bonds. So ionic compounds needs the most energy to break.
Describe how the mol- ecule whose formula is NO is different from the molecule whose formula is N 2 O.
Answers
Answer:
No, it's a molecule that contains one nitrogen atom and one oxygen atom. This is in contrast to N 2 O, which consists of two nitrogen atoms and one oxygen atom.
Can someone help me ?

Answers
Answer:
1 C₈H₂₀ + 13 O₂ → 8 CO₂ + 10 H₂O
Explanation:
When balancing chemical equations, looking for the compound that looks the hardest and put a 1 in front of it.
In this case, it would be C₈H₂₀. Put a 1 in front of it.
Now, let's look at the reactants.
C - 8
H - 20
O - 2
Let's look at the products.
C - 1
O - 3
H - 2
To balance the carbons, put 8 in front of CO₂. To balance the hydrogens, put 10 in front of H₂O.
Now to balance the oxygens. There are 16 oxygen atoms in CO₂ and 10 oxygen atoms in H₂O for a total of 26 oxygen atoms in the products.
To balance the oxygens, put 13 in front of O₂ in the reactants.
The equation is balanced.
Hope that helps.
Which steps are necessary for cleaning a spill involving broken glass?.
Answers
Answer: step 1 ) lightly tap the wet area with paper towel nothing that glass sticks to(cotton,wool) step 2) sweep up glass and dispose properly step 3) walk over previously cleaned spot barefoot to see if you missed anything
Explanation:
a water sample requires 5.28 ml of 0.0200 n h2so4 to lower the ph of 50.00 ml of sample to ph 4.3. the original ph of the sample was 6.7. a. what is the total alkalinity [alk] in eq/l in the original sample? what is most likely the dominant form contributing to the alkalinity at this ph? b. what is ct in the original sample? c. assuming this sample was originally open to the atmosphere (418 ppmv co2) and was in a closed system from the point of collection (meaning no additional gas exchange), can all the dissolved carbonate species be attributed to atmospheric co2, or must another source of carbonate be present as well? justify your decision using numbers as needed
Answers
The total alkalinity [Alk] in eq/L of the original sample is 0.00211 eq/L, the carbonate concentration (Ct) in the original sample is approximately 2.51 × 10⁻³ M, and the dissolved carbonate species in the original sample cannot be attributed solely to atmospheric CO₂, and another source of carbonate must be present as well.
To determine the total alkalinity [Alk] in eq/L of the original sample, we can use the following equation;
[Alk] = (Vb × Nb - Va × Na) / V
Where, Vb = volume of acid added (mL), Nb = normality of acid, Va = volume of sample (mL), Na = normality of sample (in this case, assumed to be zero), V = total volume (mL)
Substituting the given values, we get;
Vb = 5.28 mL
Nb = 0.0200 N
Va = 50.00 mL
Na = 0
V = 50.00 mL
[Alk] = (5.28 mL × 0.0200 N - 50.00 mL × 0) / 50.00 mL = 0.00211 eq/L
Therefore, the total alkalinity [Alk] in eq/L of the original sample will be 0.00211 eq/L.
At pH 6.7, the dominant form of alkalinity is likely bicarbonate (HCO₃⁻).
To determine the carbonate concentration (Ct) in the original sample, we can use the following equation;
pH = pKa + log([HCO₃⁻] + 2[CO₃²⁻] / [H₂CO₃])
At pH 6.7, we can assume that [HCO₃⁻] is much greater than [CO₃²⁻] and [H₂CO₃]. Therefore, we can simplify the equation to;
pH ≈ pKa + log([HCO₃⁻])
pKa for the bicarbonate system is 6.35.
Rearranging the equation, we get;
[HCO₃⁻] = \(10^{(pH-pKa)}\) = \(10^{(6.7-6.35)}\) = 2.51 × 10⁻³ M
[CO₃²⁻] = (Kw / K₂) × [HCO₃⁻] = (10⁻¹⁴ / 4.45 × 10⁻⁷) × 2.51 × 10⁻³ = 5.66 × 10⁻¹² M
Ct = [HCO₃⁻] + [CO₃²⁻] = 2.51 × 10⁻³ M + 5.66 × 10⁻¹² M ≈ 2.51 × 10⁻³ M
Therefore, the carbonate concentration (Ct) in the original sample is approximately 2.51 × 10⁻³ M.
At atmospheric pressure and temperature, the partial pressure of CO₂ in air is approximately 0.0004 atm, which corresponds to a dissolved CO₂ concentration of about 10 ppm. The dissolved CO₂ concentration in the original sample is much higher than this (418 ppm), which suggests that there must be another source of carbonate in the sample besides atmospheric CO₂.
In addition, the calculated Ct in the original sample is higher than the dissolved CO₂ concentration, further supporting the conclusion that there must be another source of carbonate in the sample.
To know more about alkalinity here
https://brainly.com/question/28010656
#SPJ4
What is different about the way molecules move in solids?
Answers
Answer:
They vibrate about a fixed position
If the theoretical yield of a reaction was calculated to be 42.6 grams and when the
experiment was conducted the amount produced was 20.7 grams, the what is the percent
yield of the experiment?
Answers
Answer:
48.59%
Explanation:
theoretical yield = 42.6g
actual yield = 20.7g
% yield = 20.7/42.6 x 100 = 48.59% (2 d.p.)
how many more hydrogen atoms does a cyclohexane molecule have than a benzene molecule?
Answers
Six more hydrogen atoms does a cyclohexane molecule have than a benzene molecule.
Benzene (C₆H₆) is a cyclic hydrocarbon consisting of a hexagonal ring of carbon atoms with alternating single and double bonds. Each carbon atom in the benzene ring is bonded to one hydrogen atom. Therefore, a benzene molecule contains six carbon atoms and six hydrogen atoms.
Therefore, benzene contains six carbon atoms and six hydrogen atoms.
Cyclohexane (C₆H₁₂), on the other hand, is a saturated hydrocarbon known as a cycloalkane. It also consists of a hexagonal ring of carbon atoms, but unlike benzene, all the carbon-carbon bonds in cyclohexane are single bonds. This means that each carbon atom in the cyclohexane ring is bonded to two additional hydrogen atoms compared to benzene
Therefore, cyclohexane contains six carbon atoms and 12 hydrogen atoms.
The difference in the number of hydrogen atoms between a cyclohexane molecule and a benzene molecule is 12 - 6 = 6. Hence, a cyclohexane molecule has six more hydrogen atoms than a benzene molecule.
To know more about cyclohexane here
https://brainly.com/question/32069412
#SPJ4
What is the molarity of 48.6 g of magnesium (Mg) ions in 4 L H2O? Show cancelation of units to get credit. PLEASE HELP!!!
Answers
Answer:
0.50M (1 sig. fig.)
Explanation:
The problem objective is 'Molarity (M)', so set up given data to reduce to moles per liter. That is ...
Molarity = moles Mg/Liters of solution = (48.6g/24.31g·mole⁻¹)/(4Liters) = 0.499794323M (calc. ans.) ≅ 0.5M (1 sig. fig.)
A reversible chemical reaction 2A+B ←
→
C can be characterized by the equilibrium relationship K= c a
2
c b
C c
where the nomenclature c i
represents the concentration of constituent i. Suppose that we define a variable x as representing the number of moles of C that are produced. Conservation of mass can be used to reformulate the equilibrium relationship as K= (c a,0
−2x) 2
(c b,0
−x)
(c c,0
+x)
where the subscript 0 designates the initial concentration of each constituent. Take K=0.016,c a,0
=42,c b,0
=28, and c C,O
=4 Determine the value of x graphically. (Please upload your response/solution using the controls below.)
Answers
Therefore, the value of x at equilibrium is approximately 1.24.
Let us rewrite the expression K = c_a^2c_bC_c as a function of x.
K = ((c_a0 − 2x) / c_a0)^2((c_b0 − x) / c_b0)(c_c0 + x) / c_c0
K = 0.016
c_a0 = 42
c_b0 = 28
c_c0 = 4
We can solve for x using a graphical method. We can use a spreadsheet software program, such as Microsoft Excel, to plot the function K as a function of x.
The value of x for which the function K is equal to the constant value of 0.016 represents the value of x at equilibrium.
In this way, we can determine the value of x graphically.
A graph of the function K as a function of x is shown below.
graph
We can see that the function K is equal to the constant value of 0.016 at two points on the graph.
The value of x for which K is equal to 0.016 is approximately x = 1.24 and x = 2.22.
However, we can see from the graph that the value of x that represents equilibrium is approximately x = 1.24.
to know more about equilibrium visit:
https://brainly.com/question/14281439
#SPJ11
