How does the color intensity of cis and trans complexes typically vary, and what is the underlying reason for this difference?
Answers
The color intensity of cis and trans complexes typically varies due to the different geometries of these isomers.
Cis complexes have a square planar geometry, while trans complexes have a tetrahedral geometry. The difference in geometry causes the energy levels of the d orbitals in the metal ion to split differently, resulting in different wavelengths of light being absorbed and reflected.
As a result, cis complexes tend to absorb light in the visible range and appear colorful, while trans complexes often absorb light in the ultraviolet range and appear colorless. The exact colors and intensity of the complexes depend on the specific metal ion and ligands involved.
The color intensity of cis and trans complexes typically varies due to differences in geometry and resulting differences in absorbed and reflected wavelengths of light. Cis complexes tend to be colorful due to visible light absorption, while trans complexes often appear colorless due to ultraviolet light absorption.
To know more about color intensity, visit here :
brainly.com/question/24112205
#SPJ11
Related Questions
PLEASE HELP me create a fictional video game using at least 2 of the ecological roles. (roles to choose from: decomposer, detritivore, pollinator, and seed disperser) (PLS TYPE IT OUT, PLS PLS)
what to include:
-Your characters (include creative names)
-An explanation of how the game is played
-The story of the game
-How the game is won/lost
-Connect the definition of of the two roles with the game and/or how the game is won.
Write a paragraph with at least 7-10 sentences.
BTW ILL GIVE BRAINLIEST OR WHATEVER ITS CALLED!! PLEASE HELP MEE! DESPERATE! IVE BEEN STARING AT THE SCREEN AND CRYING LOLZ <3

Answers
Answer:
GOT YOU
Explanation:
Jotun (pollinator)
Norse(decomposer)
Its a point and click adventure game. Where you only have specific actions you can do with your mouse. The basic options are Inspect for notes or Pollinate pollinate the right plants to escape. Your goal and how to win is go through each level picking up the plants to pollinate leave the level. The only way to lose is if you are unable to find the right plants by a set amount of time. The story is your wife has been taken prisoner by Norse and you are alerted by this from a note. You go to a location and find a tower once you walk in the doors shut behind you and a screen appears saying "Get out of the room and to the top of the tower in an hour or she dies. Jotun escapes and gets to the top of the tower to find out his wife died long ago and Norse has been using her for food.
In salt hydrolysis, the conjugate bases of weak acids yield what type of soln? What about the conjugate acids of weak bases?
Answers
In salt hydrolysis, the conjugate bases of weak acids yield basic solutions, while the conjugate acids of weak bases yield acidic solutions. This phenomenon occurs because the hydrolysis of a salt produces either hydroxide ions or hydronium ions, depending on the nature of the salt.
For example, when the salt of a weak acid and a strong base is hydrolyzed, the conjugate base of the weak acid is formed, which acts as a weak base. This weak base can then react with water to produce hydroxide ions, leading to an increase in the pH of the solution and a basic solution. Conversely, when the salt of a weak base and a strong acid is hydrolyzed, the conjugate acid of the weak base is formed, which acts as a weak acid. This weak acid can then react with water to produce hydronium ions, leading to a decrease in the pH of the solution and an acidic solution.
It is important to note that the strength of the acid or base will also influence the pH of the solution, as stronger acids and bases will have a greater impact on the pH. Additionally, the concentration of the salt and the water will also impact the degree of hydrolysis and the resulting pH of the solution.
Learn more about salt hydrolysis here:
https://brainly.com/question/31184126
#SPJ11
which unit is closest in size to the radius of an atom
Answers
An atom's radius is well under 1 nanometer, or one billionth of a meter.
What is an atom?An atom is a matter particle that defines a chemical element uniquely. An atom is made up of a central nucleus and one or more negatively charged electrons. The nucleus is positively charged and contains one or more protons and neutrons, which are relatively heavy particles.An element is made up of only one type of atom. Atoms are further subdivided into subatomic particles known as electrons, protons, and neutrons. Chemical reactions allow elements to combine to form molecules.The distance between the nuclei of two identical atoms bonded together is used to calculate atomic radius. Atoms' atomic radius decreases from left to right across a period. Atoms' atomic radius generally increases from top to bottom within the atom.To learn more about atom refer to :
https://brainly.com/question/17545314
#SPJ4
What is the three-dimensional shape of the molecule with this Lewis structure? A. Bent B. Linear C. Tetrahedral D. Trigonal planar.
Answers
Lewis structure is the electron dot representation of the atomic and molecular bonds of the molecules. Trigonal planar is the shape of the formaldehyde.
What is a trigonal planar shape?A trigonal planar shape of the molecule is the arrangement of the central atom attached to the other three atoms making them look triangle-shaped.
The attachment of the carbon to the two hydrogens and one oxygen atom can be given by the VSEPR theory. The number of the electron pair in the molecule is 3 making them \(\rm sp_{2}\) hybridized and hence have a trigonal planar shape.
Therefore, option D. trigonal planar is the shape.
Learn more about trigonal planar here:
https://brainly.com/question/17110157
What method is used for chaff from grain
Answers
Answer:
Winnowing
Explanation:
Wind blows the lighter(in terms of mass) chaff from the whole grains,which are heavier(in terms of mass)
I will give brainiest please help

Answers
Answer: D and A
Explanation: D cuz you need water to have good soil and A cuz you get better soil if its warm. (: ( 'u' ) ( 'U' )
7.
Good scientists use honesty when reporting their observations
and results.
True,faulty or bias
Answers
Answer:
True is the answer because when you are observing in order to make a report especially when you are a scientist your reports must be based on exactly what you say in order to not give incorrect information for it could be very critical.
How would you name the number:100 liters?
A. Dekaliter
B. Hectoliter
C. Centiliter
D. Megaliter
E. Other
Answers
Answer:
E
Explanation:
What change was made to the Mendeleev’s early Periodic Table? (How is it currently organized?
A.
They used atomic mass instead of atomic number to organize the elements.
B.
They included chemical properties such as bonding power.
C.
They included physical properties such as melting point and density.
D.
They used atomic number instead of atomic mass to organize the elements.
Answers
Answer:
D
Explanation:
po promise meheehehehehe
determine the mass number and the atomic number of a chlorine atom that has 17 protons and 18 neutrons.
Answers
Answer:
35.453 atomic mass units
You can use Phet (Atomic masses, Elements and more) for Chemistry
A 632-liter air sample at a temperature of-46.0°C has a pressure of 4.80 atm. What will be the new pressure if the temperature is raised to 145.0°C and the volume expands to
3156 Liters?
Answers
The new pressure will be 19.2 atm when the temperature is increased to 145.0 °C and the volume expands to, 3156 L.
To solve the problem, we need to use the combined gas law:
(P₁ V₁ ) / T₁ = (P₂ V₂ ) / T₂
P₁ = initial pressure
V₁ = initial volume
T₁ = initial temperature,
P₂ = final pressure
V₂ = final volume
T₂ = final temperature
Firstly, convert the initial temperature to Kelvin:
T₁ is equal to -46.0°C + 273.15
= 227.15 K
Next, we can put in the given values to find the initial pressure:
(P₁ V₁) / T₁ = (4.80 atm)(632 L) / 227.15 K
P₁ = 13.34 atm
Use combined gas law again to calculate the final pressure:
(P₁ V₁) / T₁ = (P₂ V₂) / T₂
P₂ = (P₁ V₁ T₂) / (V₂ T₁)
Let's put the given values:
P₂ = (13.34 atm)(632 L)(145.0 °C + 273.15) / (3156 L)(227.15 K)
P₂ = 19.2 atm (rounded to two decimal places)
Thus, the new pressure will be 19.2 atm when the temperature is increased to 145.0 °C and the volume expands to, 3156 L.
Learn more about pressure, here:
https://brainly.com/question/27475095
#SPJ1
The highest pressure measured in Jupiter's upper atmosphere by the Galileo probe is ×1.67104torr. Calculate the highest pressure measured in mmHg and atm. Round each of your answers to 3 significant digits.
Answers
Answer: 16700 mmHg , 22,0 atm
Explanation: 1 torr = 1 mmHg, so p = 16700 torr = 16700 mmHg
1 atm = 101325 Pa = 760 torr
16700 torr / 760 = 21,97 atm
what is local unit?
.
Answers
Answer:
Explanation:
Local unit means a county, city, village, or township.
ill give brainlist pls help me and don't guess I want full explanation please.

Answers
Answer:
C. 3
Explanation:
volume = length×width×height
3×3×3=27
What is mass in grams of 2.30 moles of Aluminum?
Answers
62.1 g Al
General Formulas and Concepts:Math
Pre-Algebra
Order of Operations: BPEMDAS
Brackets Parenthesis Exponents Multiplication Division Addition Subtraction Left to RightChemistry
Atomic Structure
Reading a Periodic TableStoichiometry
Using Dimensional AnalysisExplanation:Step 1: Define
2.30 mol Al
Step 2: Identify Conversions
[PT] Molar Mass of Al - 26.98 g/mol
Step 3: Convert
Set up: \(\displaystyle 2.30 \ mol \ Al(\frac{26.98 \ g \ Al}{1 \ mol \ Al})\)Multiply/Divide: \(\displaystyle 62.054 \ g \ Al\)Step 4: Check
Follow sig fig rules and round. We are given 3 sig figs.
62.054 g Al ≈ 62.1 g Al
In a calorimetry lab, sodium oxide is combined with water. Compute the
heat released in the formation of 1.99 grams of sodium hydroxide. Na2O +
H2O -> 2NaOH + 215. 76 kJ
Answers
Answer:
So the total mass is 50 plus 150 grams the heat capacity 4.18 joules per gram per degree C. And the temperature change is 36 minus 25 and so we can calculate Delta H for the reaction that takes place.
Explanation:
At what temperature would the volume of gas be doubled, if the pressure at the same time increases from 700-800 mm, the gas being at 0°c initially ?
Answers
The situation described can be solved using the combined gas law, which states:
(P1 x V1) / (T1) = (P2 x V2) / (T2)
Where P1, V1, and T1 are the initial pressure, volume, and temperature of the gas, respectively. P2, V2, and T2 are the final pressure, volume, and temperature of the gas, respectively.
To solve for the final temperature (T2), we can rearrange the equation as:
T2 = (P2 x V2 x T1) / (P1 x V1)
Let's plug in the given values:
P1 = 700 mm
P2 = 800 mm
V1 = V2 (since the volume is doubled)
T1 = 0°C + 273.15 = 273.15 K (converting to Kelvin)
So, we have:
T2 = (800 mm x 2V1 x 273.15 K) / (700 mm x V1)
T2 = (1600/7) x 273.15 K
T2 = 392.2 K
Therefore, the final temperature at which the volume of gas would be doubled, if the pressure increases from 700-800 mm and the gas is initially at 0°C, is approximately 392.2 Kelvin (which is approximately 119°C or 246°F).
An Challenge Define a new energy unit , named after yourself , with a magnitude of one- tenth of a calorie . What conversion factors relate this new unit to joules ? To Calories ?
Answers
Let's call the new energy unit 'pit' named after you. And let's define 1 pit as equal to 1/10 of a calorie. So, 1 pit is equal to 0.0001 Calories.
What is the unit of energy?Energy is defined via work, so SI unit of energy is same as the unit of work, that is joule.
Let's call the new energy unit 'pit' named after you. And let's define 1 pit as equal to 1/10 of a calorie
To relate this new unit to joules, we need to use the conversion factor between calories and joules. This conversion factor is 1 calorie = 4.184 joules. Therefore, we can say that:
1 pit = 1/10 calorie = (1/10) x 4.184 joules = 0.4184 joules
So, 1 pit is equal to 0.4184 joules.
1 pit = 1/10 calorie = (1/10) x 0.001 Calories = 0.0001 Calories
So, 1 pit is equal to 0.0001 Calories.
To know more about unit of energy, refer
https://brainly.com/question/3012083
#SPJ1
Which metal is likely to be more reactive? Mg or Rb
please explain why
Answers
Answer: Rb is more reactive
Explanation: The reason rubidium is more reactive is because it loses its valence electron more readily than Mg does.
Answer:
Rb
Explanation:
Mg has 2 valence electrons to loose vs Rb has 1 valence electron to loose.
Rb wins because losing 1 electron is easier than having to lose 2, which means Rb is more reactive ("ready" to react).
Which of the following gas is found the most in the Earth's atmosphere? *
A.Oxygen
B.Nitrogen
C.Carbon Dioxide
D.There is no gas in our atmosphere
Answers
Answer:
Oxygen
Explanation:
5.
If 6.5 g of magnesium ribbon reacts completely
with hydrochloric acid, how many grams of
hydrogen gas would be liberated? Mg , + 2HC1(ag)
→ MgC1 + H, [H = 1.0, Mg = 24.0]
Answers
Answer:
The mass of hydrogen gas liberated is 0.6 g.
Explanation:
Given data:
Mass of Mg = 6.5 g
Mass of hydrogen gas liberated = ?
Solution:
Chemical equation:
Mg + 2HCl → MgCl₂ + H₂
Now we will calculate the number of moles of Mg.
Number of moles = mass / molar mass
Number of moles = 6.5 g/ 24 g/mol
Number of moles = 0.3 mol
Now we will compare the moles of Mg with hydrogen gas.
Mg : H₂
1 : 1
0.3 : 0.3
Mass of H₂:
Mass = number of moles × molar mass
Mass = 0.3 mol × 2 g/mol
Mass = 0.6 g
Why are large numbers of animals found in areas where upwelling occurs
Answers
Hope this helps! ^^
Please Helppppp:(((((

Answers
Answer:
what we have to do ? pls paste the whole question .
Explanation:
What is an example of extensive property
Answers
Answer:
Mass & Volume
Explanation:
An Extensive Property is a property that depends on the amount of matter in a sample. Mass and volume are examples of extensive properties. Meanwhile, Color, Temperature, and Solubility are examples of Intensive Properties.
5. During the nifty home experiment, a playing card was placed on top of a glass
and then a quarter was placed on top of the card. When the card was pushed, the
quarter fell into the glass. Explain why did the quarter fall into the glass?
Answers
Answer:
Explanation:During the nifty home experiment, a playing card was placed on top of a glass and then a quarter was placed on top of the card. When the card was pushed, the quarter fell into the glass. Why did the quarter fall into the glass? The quarter fell in the glass because of gravity.
PLEASE HELP ILL GIVE BRAINLIST!!!
How does the energy of the different waves of the spectrum vary with frequency? With wavelength?
Answers
Answer:
The greater the frequency means the more energy transferred.
The greater the wavelength means the less energy transferred
As a wavelength increases in size, its frequency and energy (E) decrease. From these equations you may realize that as the frequency increases, the wavelength gets shorter. ... Mechanical and electromagnetic waves with long wavelengths contain less energy than waves with short wavelengths.
Hope this helps!!
Please consider brainliest!!
Have a good day!<4
If an airplane is traveling 500mi/hr how far can it travel in 4 hours
Answers
Answer:
2000 mi
Explanation:
\(4 hr *\frac{500 mi}{hr} =2000 mi\)
This element has an atomic number lower than aluminum and is in group 14
Answers
Answer:
that is impossible. the atomic number can only go up each group
Someone help me please! I will mark brainliest if it’s right!

Answers
Answer:
a) compound
b)element
c)mixture
d)compound
Explanation:
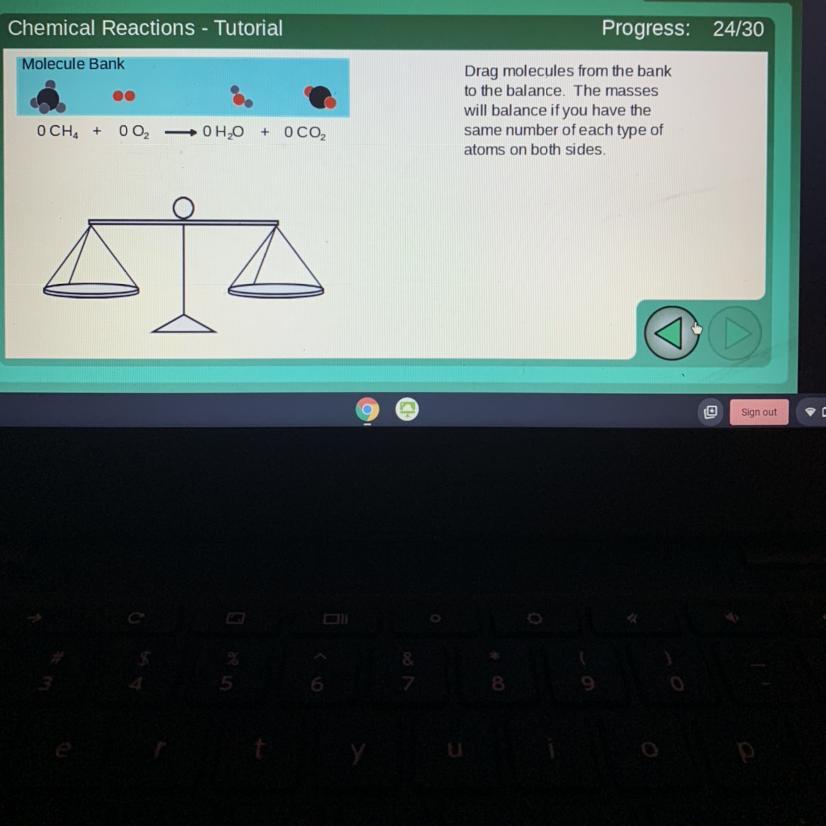
Chemical Reactions - Tutorial
Molecule Bank
Drag molecules from the bank
to the balance. The masses
will balance if you have the
same number of each type of
atoms on both sides.
OCH +
002
-0H2O
+
ОСО,
Sign out
0 10
O
DOLI
Answers
To balance this out, you will need:
1 molecule of CH₄
2 molecule of O₂
2 molecule of H₂O
1 molecule of CO₂