I will give brailiest to whoever needs it!
Answers
Answer:
pls pls pls pls pls give me brainliest i just need one more plssssssss
Explanation:
Related Questions
HELP ME WITH THIS PLSSSSS

Answers
Answer:Predatorrrrrrrrrrrrrrrr
Pls gimme Brainliest
DOES ANYONE KNOW ANY OF THE ANSWERS TO THIS ??

Answers
Answer:
Which one do you need the answer to?
Explanation:
PLEASE ANSWER!!!!! 35 POINTS!!
How many moles of P2O3 are required to fully react with 108 H2O? (H2O; 18 g/mol)
P2O3 + 3H2O --> 2H3PO3
108 gH2O ---> mol P2O3
Answers
108 g H2O x (1 mol H2O / 18 g H2O) = 6 moles H2O
According to the balanced chemical equation, 3 moles of H2O react with 1 mole of P2O3 to produce 2 moles of H3PO3. Therefore, you need to divide the number of moles of H2O by 3 and multiply by 2 to get the number of moles of H3PO3 required:
6 moles H2O / 3 x 2 moles H3PO3 / 1 mole P2O3 = 4 moles P2O3
So, you would need 4 moles of P2O3 to fully react with 108 g of H2O.
For 2H₂ + O₂ → 2H₂O:
4 moles of H₂ will react with
moles of O₂ to produce
moles of H₂O

Answers
Answer:
in this reaction, 4 moles of H₂ will react with 2 moles of O₂ to produce 4 moles of H₂O.
Explanation:
The balanced equation 2H₂ + O₂ → 2H₂O tells us that 2 moles of hydrogen gas (H₂) will react with 1 mole of oxygen gas (O₂) to produce 2 moles of water (H₂O).
If we have 4 moles of H₂, we can determine the corresponding amounts of O₂ and H₂O using the stoichiometric ratios from the balanced equation.
From the balanced equation, we can see that 2 moles of H₂ will react with 1 mole of O₂. Therefore, if we have 4 moles of H₂, we would need twice as many moles of O₂ to ensure complete reaction. Thus, we would require 2 moles of O₂.
Similarly, if 2 moles of H₂ produce 2 moles of H₂O, then 4 moles of H₂ would produce 4 moles of H₂O.
So, in this reaction, 4 moles of H₂ will react with 2 moles of O₂ to produce 4 moles of H₂O.
Which of the following flows is not driven by pressure differences?
1: A deep breath
2: A river
3: A gust of wind
4: A sip through a drinking straw
Answers
Answer:
I think it is option (b) river
The river flows is not driven by pressure differences.
The wind often blows due to of differences in air pressure from one location to another. Wind blows often from zones of high pressure toward zones of low pressure and thus when the high pressure point is very close to the low pressure point the wind can blow very fast.But the river is not driven by pressure differences as it flows in its course.Conclusively we can say that the river flows is not driven by pressure differences
Learn more from:
https://brainly.com/question/10152119
Calcium carbonate decomposes at 832oC in the following equation: CaCO3(s) → CaC(s) + CO2(g). The reaction is first order with a rate constant of 2.66 x 10-3/s at 832oC. How long will it take for the reaction to produce 76.0% product?
Answers
The time taken to produce 76.0% of the product is 150 seconds
Given: Calcium carbonate decomposes at 832°C in the following equation: CaCO3(s) → CaC(s) + CO2(g).
The reaction is first order with a rate constant of 2.66 x 10-3/s at 832°C. To find the time taken to produce 76.0% product.Solution: We know that the reaction is first order.
So, we can use the first-order integrated rate law to find the time taken to produce a 76.0% product.
The first-order integrated rate law equation is given as follows: ln(Aₙ/A₀) = - kt
Here, [A]t = concentration of A at time t
A₀ = initial concentration of Ak = rate constant = time
Now, we can find the concentration of A at time t, which is 76.0% of the initial concentration A₀= 100 - 76.0% = 24.0% = 0.24
[A]t/[A]0 = 0.76/1 = 0.76
Substituting these values in the first-order integrated rate law equation, we get:
ln(0.76) = - (2.66 × 10-3/s) × t
t = ln(0.76) / (2.66 × 10-3/s)
t = 150.37 s ≈ 150
Therefore, the time taken to produce 76.0% product is 150 seconds (approximately).Answer: The time taken to produce 76.0% of the product is 150 seconds (approximately).
To learn about the rate law equation here:
https://brainly.com/question/16981791
#SPJ11
How many moles of hydrochloric acid are in 500. Ml of a 0. 300 m solution?.
Answers
Answer:
300
Explanation:
because it's not highly concentrated
Q1:a) for the following mechanism:
Step 1: NO₂(g)+NO₂(g) →NO(g) +NO3(g) Step 2: NO3(g) + CO (g) NO₂ (g) + CO₂(g)
Experimentally the rate law is found to be: Rate = k[NO₂]².
1-Write the equation for the overall reaction:
2-Identify the intermediate: (slow) (fast)
3- What is the molecularity of the rate-determining step?
Answers
Answer:
1-The overall reaction is the combination of the two steps, so the equation for the overall reaction can be written as:
NO₂(g) + NO₂(g) + CO(g) → NO₂(g) + CO₂(g)
2-The intermediate is the species produced in the first step and consumed in the second step, so it can be identified as:
NO3(g)
3-The rate-determining step is the slowest step in the reaction mechanism, so the molecularity of the rate-determining step is:
2 (because the rate law is found to be Rate = k[NO₂]², which implies that the rate is dependent on the concentration of two NO₂ molecules)
Explanation:
Just tell me if you kinda confuse
ALLEN
Identify at least two physical properties (streak, fracture, etc.) of a mineral while using examples of common minerals that prominently feature those properties. Provide links or screenshots of the discussed minerals to illustrate the highlighted properties. Also, explain what mineral group it belongs to and why.
Answers
The two physical properties of a mineral can be color, streak and even cleavage also.
Color, streak, cleavage, hardness, specific gravity, fracture, luster, and crystal structure are only a few of the many diverse characteristics. I'll speak specifically about color and shine as they relate to diamonds and how they significantly impact the value of the individual stone. Diamonds are a type of carbon polymorph.
Polymorphism, as used in materials science, refers to the fact that a solid material can exist in more than one crystal structure or form. Isomerism in the form of polymorphism. The phenomena can be seen in any crystalline substance. A chemical element's polymorphism is referred to as allotropy. Pharmaceuticals, agrochemicals, pigments, dyestuffs, meals, and explosives all have practical applications for polymorphism. "A reversible transition of a solid crystalline phase at a given temperature and pressure (the inversion point) to another phase of the same chemical composition with a different crystal structure," according to IUPAC, describes a polymorphic transition.
Learn more about physical properties
brainly.com/question/18327661
#SPJ4
How many grams of sodium hydroxide are in 2.5 liters of a 4 M solution?
Answers
- Which equation represents the radioactive decay
of 226Ra?
(1) Ra→ 86
226Ra89
(2)
(3) 88
226
(4) Ra→Ra+n
88
88
226 Ra
.
222Rn+He
226 Act
226 Fr+
87
0
ve
0
the
Answers
The correct equation representing the radioactive decay of 226Ra is (2) 226Ra → 222Rn + 4He.
This equation correctly represents the radioactive decay process of 226Ra (Radium-226) into 222Rn (Radon-222) and 4He (Helium-4).
In radioactive decay, unstable isotopes undergo a spontaneous process to transform into more stable forms by emitting particles or radiation. In the case of 226Ra, it decays by a process called alpha decay. Alpha decay involves the emission of an alpha particle, which consists of two protons and two neutrons (equivalent to a helium nucleus).
The equation (2) shows that 226Ra undergoes alpha decay, resulting in the formation of 222Rn and the release of an alpha particle (4He). The atomic number and mass number must be conserved in the decay process. Therefore, 226 (atomic number 88) on the left side of the equation decays to 222 (atomic number 86) on the right side, while the mass number also decreases by 4 units.
It's important to note that equation (1) represents an incorrect notation, as it suggests that 226Ra undergoes a transformation to 226Ra89, which is not possible as it implies an increase in atomic number. Equation (3) does not accurately represent the decay of 226Ra. Equation (4) is incorrect as it represents the formation of an isotope that doesn't exist.
for more such questions on radioactive
https://brainly.com/question/18640165
#SPJ8
Which statement best compares pure substances to mixtures?
Pure substances contain small amounts of impurities. Mixtures contain large amounts of
impurities.
Pure substances must be made of two chemical materials. Mixtures can be made of only one
chemical material.
Pure substances are either compounds or elements. Mixtures are homogeneous or
heterogeneous combinations.
Pure substances are liquids dissolved in liquids or gases dissolved in gases. Mixtures are
gases dissolved in liquids or solids dissolved in liquids.
Answers
Answer:
pure substances contain small amount of impurities . mixture contain large amounts of impurities
Pure substances are either elements or compounds while mixtures are homogeneous or heterogeneous combinations.
What is Pure substance and mixture?Elements are pure substances as they cannot be broken down into simple substances by any chemical or physical methods as they have only one type of atom in the whole composition.
A pure substance is composed of two or more elements that are chemically combined in a definite proportion. That type of pure substance is called a compound. Water is a pure compound made up of a combination of oxygen and hydrogen.
A mixture is composed of two or more different types of substances which are physically combined. A mixture can separate back into its original components. These types of impure substances are also referred to as mixtures.
A mixture can be either homogeneous or heterogeneous. Cement is a calcium compound solid it is considered a homogeneous mixture while famous gaseous heterogeneous mixes such as cologne and perfume.
Therefore, statement (C) best compares pure substances to mixtures.
Learn more about pure substances and mixtures, here:
https://brainly.com/question/6243623
#SPJ2
a material is made of molecules of mass 3.0×10−26kg3.0×10−26kg. there are 6.1×10286.1×1028 of these molecules in a 2.0-m3m3 volume. What is the density of the material?
Answers
The density of the material is approximately 9.15 × 10² kg/m³. The density of a material is defined as its mass per unit volume.
To calculate the density of the given material, we can divide the total mass of the molecules by the volume.
Given:
Mass of each molecule = 3.0 × 10⁻²⁶ kg
Number of molecules = 6.1 × 10²⁸
Volume = 2.0 m³
To calculate the total mass, we can multiply the mass of each molecule by the number of molecules:
Total mass = (3.0 × 10⁻²⁶ kg/molecule) × (6.1 × 10²⁸ molecules)
To calculate the density, we can divide the total mass by the volume:
Density = Total mass / Volume
Let's perform the calculations:
Total mass = (3.0 × 10⁻²⁶kg/molecule) × (6.1 × 10²⁸ molecules)
Density = Total mass / Volume
Density = [(3.0 × 10⁻²⁶ kg/molecule) × (6.1 × 10²⁸ molecules)] / (2.0 m³)
Now let's simplify the expression:
Density = (3.0 × 6.1) × (10⁻²⁶ × 10²⁸) / 2.0
Density = 18.3 × 10² / 2.0
Density = 9.15 × 10² kg/m³
Therefore, the density of the material is approximately 9.15 × 10²kg/m³.
To learn more about density here
https://brainly.com/question/29775886
#SPJ4
perform the following operation
and express the answer in
scientific notation.
5.4x103 x 1.2x107
Answers
Answer:
(5.4 x 10³) x (1.2 x 10⁷) = 6.48 x 10¹⁰
With correct significant figures, the answer would be 6.5 x 10¹⁰.
Plz plz here
A student pours 25.0 mL of water into a graduated cylinder. She drops a plece of unknown metal with a mass of 18.9 g Into the cylinder. The
water level rises to 32.0 mL. The student uses the table to identify the metal
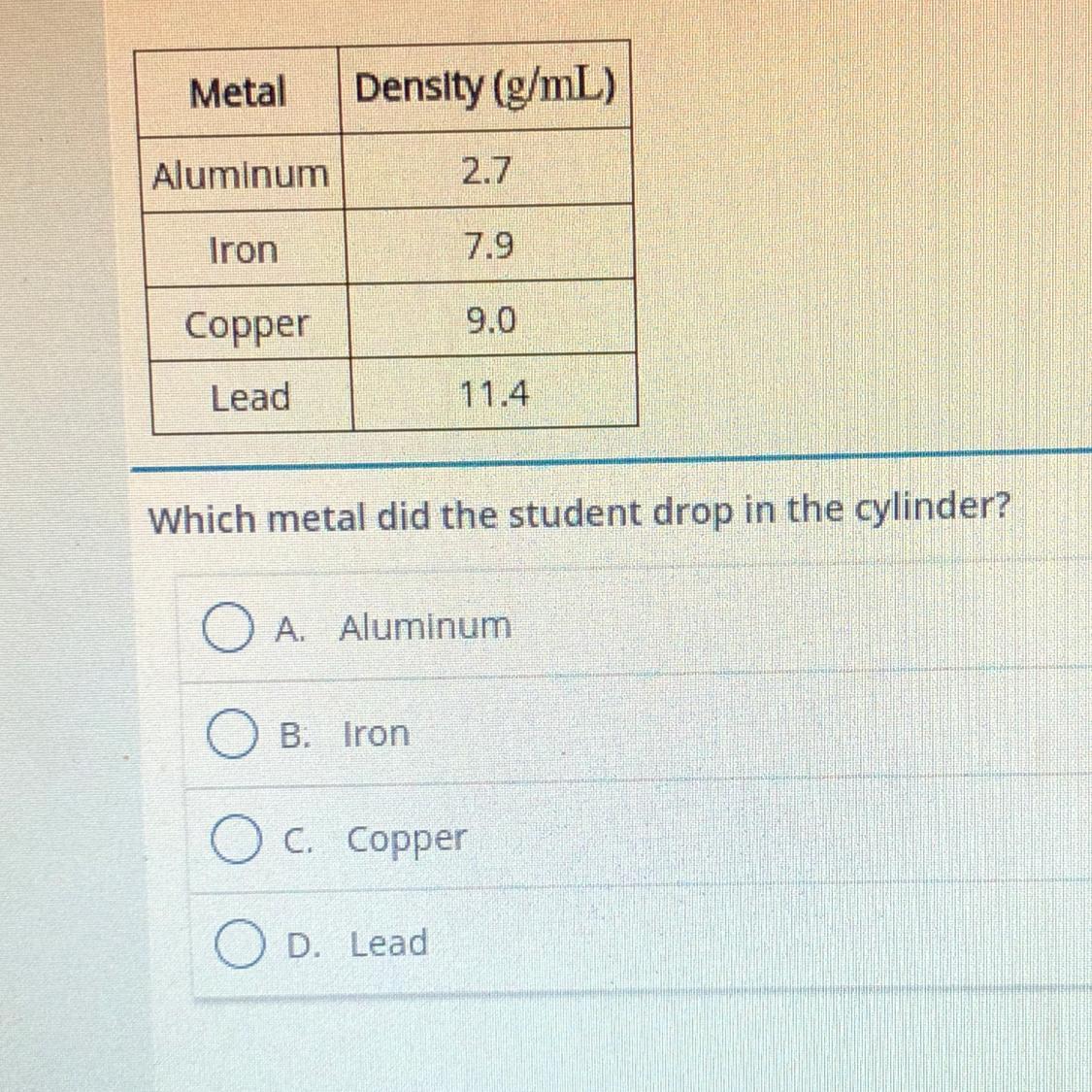
Answers
Answer:
Aluminum
Explanation
The volume of the metal is found by subtracting the final volume by the Intel volume giving 7
And the formula for density is mass divided by volume hence minus 7 by 18.9 giving 2.7
Silver has two stable isotopes. The nucleus, 10747Ag, has atomic mass 106.905095 g/mol with an abundance of 51.83% ; whereas 10947Ag, has atomic mass 108.904754 g/ mol with an abundance of 48.17%. What is the binding energy per nucleon for each isotope?
Answers
To calculate the binding energy per nucleon for each isotope of silver, we need to determine the total binding energy of each isotope and divide it by the number of nucleons (protons and neutrons) in each isotope.
The binding energy per nucleon represents the average energy required to remove a nucleon from the nucleus. It is a measure of the stability of the nucleus. To calculate the binding energy per nucleon, we need to determine the total binding energy and the number of nucleons for each isotope. First, we calculate the total binding energy for each isotope using the mass-energy equivalence equation E = mc², where E is the energy, m is the mass, and c is the speed of light.
For the first isotope, 10747Ag, with an atomic mass of 106.905095 g/mol, we multiply the atomic mass by the Avogadro's number to obtain the mass of one mole of atoms. Then we divide the mass by the number of atoms in one mole to get the mass of one atom. The difference between the mass of one atom and the mass of the individual nucleons (protons and neutrons) gives the mass defect. Multiplying the mass defect by c² gives the binding energy of one atom. Finally, dividing the binding energy by the number of nucleons in the isotope gives the binding energy per nucleon.
We repeat the same calculations for the second isotope, 10947Ag, with an atomic mass of 108.904754 g/mol. The resulting values represent the binding energy per nucleon for each isotope of silver.
Learn more about isotope here: https://brainly.com/question/20596678
#SPJ11
What causes the periodic change between night and day?
Earth's revolution around the sun
the moon's rotation
the moon's revolution around Earth
Earth's rotation
Answers
Answer:
Earths Rotation
Explanation:
Answer:
D
Earth's rotation
Explanation:
Mark as branliest if right but im pretty sure im right
The velocity of sound in water is 350 m/s. If a wave eith a wavelength of 6 m travels water, at what frequency will it be traveling?
Answers
Answer: 1,480m/s
Explanation:
in a nuclear fusion reaction, which has more mass: the initial hydrogen isotopes or the fusion products?
Answers
the initial hydrogen isotopes in a nuclear fusion reaction have more mass than the fusion products.
Powering the Sun and other stars are nuclear fusion process. Two light nuclei combine to form one heavy nucleus during a fusion process. Because the mass of the single nucleus formed is less than the combined mass of the two initial nuclei, energy is released throughout the process. Energy is created from the remaining mass. The reason this happens is explained by Einstein's equation (E=mc2), which says that mass and energy can be transformed into one another. Fusion energy could play a significant role in the creation of energy if scientists can figure out how to use it in Earth-based machinery. Numerous elements from the periodic table can undergo fusion. However, the deuterium-tritium (DT) fusion reaction is of particular interest to scientists studying fusion energy applications. DT fusion generates a neutron and a helium nucleus.
learn more about nuclear fusion here;
https://brainly.com/question/17870368
#SPJ4
Partial bonding, for example, as part of a resonance hybrid, often results in structures with _____.
Answers
Answer:
fractional bond orders
Explanation:
because fractional bond orders
What type of weathering will occur more rapidly in an area with extremely cold winters and hot summers?
Answers
Answer:
mechanical weathering through the process of ice wedging
Explanation:
Answer:
In cold climates, the freezing and thawing that occurs causes rapid mechanical weathering through the process of ice wedging. In warmer climates, chemical weathering is more rapid because the chemical reactions that dissolve rocks and minerals are accelerated by warm temperatures.
Explanation:
:)
What is the electronegativity periodic table?
Answers
The electronegativity periodic table is a chart that arranges elements according to their electronegativity, which is a measure of an atom's ability to attract electrons to itself. Electronegativity is a chemical property that reflects the relative tendency of an atom to draw electrons towards itself when it forms a chemical bond with another atom.
The electronegativity values are usually determined using the Pauling scale, which was developed by Linus Pauling and is widely used in chemistry. In this scale, the electronegativity of an element ranges from 0.7 for cesium to 4.0 for fluorine, with increasing electronegativity moving from left to right across a period and increasing as one moves down a group.
The electronegativity values can be useful in understanding chemical bonding and the behavior of molecules. For example, elements with high electronegativity values tend to form ionic bonds, while elements with low electronegativity values tend to form covalent bonds. Additionally, the electronegativity difference between two bonded atoms determines the type of bond, with larger differences indicating polar covalent bonds and smaller differences indicating nonpolar covalent bonds.
To know more about electronegativity click here:
https://brainly.com/question/17762711#
#SPJ11
WILL GIVE BRAINLIEST FOR THE RIGHT ANSWER!!!!!Which elements can form diatomic molecules joined by a single covalent bond?
A. hydrogen only
B. halogens only
C. halogens and members of the oxygen group
D. hydrogen and the halogens only
Answers
Answer:
b
Explanation:
that's the answerer
During a process called photoact, ________ give up an electron as a part of the light-dependent reactions.
Answers
Answer:
Chloroplasts?
Explanation:
E° (V)
Al3+ + 3e- → Al -1.66
Ni2+ + 2e- → Ni -0.23
If this cell is set up at 25°C with [Ni2+] = 1.00 × 10-3M and [Al3+] = 2.00 × 10-2M, the expected cell potential is what?
Answers
The expected cell potential is 1.43 V when this cell is set up at 25°C with [Ni2+] = 1.00 × 10-3M and [Al3+] = 2.00 × 10-2M, assuming standard conditions and perfect mixing.
To calculate the expected cell potential, we need to use the equation: cell potential = E°(cathode) - E°(anode)
where E° is the standard reduction potential for each half-cell reaction.
From the given half-cell reactions:
Al3+ + 3e- → Al E° = -1.66 V
Ni2+ + 2e- → Ni E° = -0.23 V
The reaction with the higher reduction potential is the reduction of Ni2+ to Ni, so this reaction will occur at the cathode. Therefore, we have:
E°(cathode) = -0.23 V
E°(anode) = -1.66 V
Plugging in the values, we get:
cell potential = -0.23 V - (-1.66 V)
cell potential = 1.43 V
Learn more about cell potential here:
https://brainly.com/question/14390070
#SPJ11
at 25 °c, how many dissociated h ions are there in 325 ml of an aqueous solution whose ph is 11.41? number of h ions:
Answers
Aat 25 °C, the number of dissociated H+ ions there are in 325 ml of an aqueous solution whose pH is 11.41 is 1.235 × 10⁻¹².
The pH scale is a logarithmic measure of the acidity or basicity of an aqueous solution. The pH scale ranges from 0 to 14, with 7 being neutral, below 7 being acidic, and above 7 being basic or alkaline. Therefore, we can use the relationship between pH and [H+] concentration to calculate the number of dissociated H+ ions in an aqueous solution. The relationship is as follows:
pH = -log[H+]
or [H+] = 10^-pH
We can use this relationship to find the number of dissociated H+ ions in 325 mL of an aqueous solution whose pH is 11.41 as follows:
First, we need to find the [H+] concentration from the given pH:
[H+] = 10^-pH = 10^-11.41 = 3.80 × 10⁻¹² M
Now that we know the [H+] concentration, we can calculate the number of dissociated H+ ions using the following formula:
Number of dissociated H+ ions = [H+] × volume of solution in Liters
The volume of the solution is given in milliliters (mL), so we need to convert it to liters (L):
325 mL = 325/1000 L = 0.325 L
Now we can use the formula above to find the number of dissociated H+ ions:
Number of dissociated H+ ions = [H+] × volume of solution in Liters = 3.80 × 10⁻¹² M × 0.325 L = 1.235 × 10⁻¹² mol
The answer is: 1.235 × 10⁻¹² dissociated H+ ions.
Learn more about pH scale here: https://brainly.com/question/26424076
#SPJ11
How much heat is needed to raise a 0.060 kg piece of granite from 82°C to 212 °C.
(The specific heat capacity of granite is 0.790
Answers
Answer:
There are required 6162J to raise the temperature of the piece of granite from 82°C to 212°C
Explanation:
By using the equation:
Q = m×C×ΔT
Where Q is heat in J, our incognite
m is mass of the substance, 0.0600kg = 60.0g
C is specific heat, 0.790J/g°C
And ΔT is change in temperature, 212°C - 82°C = 130°C
Replacing:
Q = m×C×ΔT
Q = 60.0g×0.790J/g°C×130°C
Q = 6162J
There are required 6162J to raise the temperature of the piece of granite from 82°C to 212°C
Are the following chemical equations reversible or irreversible?
2H2O ←→ H3O+ + OH-
HA + H2O ←→ A- + H3O+
HA + H2O → A- + H3O+
MOH → M+ + OH-
Answers
The first two chemical equations are reversible while the other two are irreversible.
What are chemical equations?Chemical equation is a symbolic representation of a chemical reaction which is written in the form of symbols and chemical formulas.The reactants are present on the left hand side while the products are present on the right hand side.
A plus sign is present between reactants and products if they are more than one in any case and an arrow is present pointing towards the product side which indicates the direction of the reaction .There are coefficients present next to the chemical symbols and formulas .
The first chemical equation was put forth by Jean Beguin in 1615.By making use of chemical equations the direction of reaction ,state of reactants and products can be stated. In the chemical equations even the temperature to be maintained and catalyst can be mentioned.
Learn more about chemical equations,here:
https://brainly.com/question/19626681
#SPJ1
A physics experiment is conducted at a pressure of 14.4 kpa. what is this pressure in mmhg?
Answers
A pressure of 14.4 kPa is equal to 108.007 mmHg.
The pressure of 14.4 kPa can be converted to mmHg using the conversion factor of 7.50062 mmHg/kPa.
To convert kPa to mmHg, you can multiply the given pressure by the conversion factor:
14.4 kPa * 7.50062 mmHg/kPa = 108.007 mmHg.
Therefore, the pressure of 14.4 kPa is equal to 108.007 mmHg.
In physics, pressure is a measure of the force applied per unit area. The units of pressure can vary depending on the system being used. In this case, kPa stands for kilopascals, which is a metric unit of pressure commonly used in scientific calculations. On the other hand, mmHg stands for millimeters of mercury, which is a unit of pressure often used in the field of medicine.
To convert from kPa to mmHg, we need to use the conversion factor of 7.50062 mmHg/kPa. This conversion factor represents the ratio between the two units of pressure. Multiplying the given pressure of 14.4 kPa by the conversion factor gives us the equivalent pressure in mmHg, which is 108.007 mmHg.
So, in summary, a pressure of 14.4 kPa is equal to 108.007 mmHg.
Learn more about pressure here:-
https://brainly.com/question/29341536
#SPJ11
Is hydrogen bond an attraction between polar molecules
Answers
Answer:
No because if there was an attraction hydrogen would not do it's function