"Identify each of the solutions as acidic, basic, or neutral. [oh−]=1.0×10^−7M [oh−]=1.0×10^−9M[h3o+]=0.0001M[h3o+]=7.3×10^−3M"
Answers
[oh−]=1.0×10^−7M and [h3o+]=7.3×10^−3M are acidic solutions, [oh−]=1.0×10^−9M is a basic solution, and [h3o+]=0.0001M is a neutral solution.
The explanation for this answer is that the pH scale ranges from 0 to 14, with 7 being neutral. A pH below 7 is considered acidic, while a pH above 7 is considered basic.
The [oh−] and [h3o+] concentrations can be used to calculate the pH of a solution using the formula pH=-log[H+].
In the case of these solutions, the pH values are as follows: [oh−]=1.0×10^−7M has a pH of 7.0, [oh−]=1.0×10^−9M has a pH of 9.0, [h3o+]=0.0001M has a pH of 4.0, and [h3o+]=7.3×10^−3M has a pH of 2.14.
In summary, the [oh−]=1.0×10^−7M and [h3o+]=7.3×10^−3M solutions are acidic, [oh−]=1.0×10^−9M is basic, and [h3o+]=0.0001M is neutral. The pH values were calculated using the concentrations of [oh−] and [h3o+], and the pH scale was used to determine if the solutions were acidic, basic, or neutral.
Learn more about acidic solutions click here:
https://brainly.com/question/24255408
#SPJ11
Related Questions
what does combustion mean
Answers
Answer:
combustion is a high-temperature exothermic redox chemical reaction between a fuel and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke.
a solution is made by mixing 9.76 ml of 4.00 m acetone, 4.11 ml of 1.00 m hcl, 10.00 ml of 0.0500 m i2 and 20.00 ml of water. what is the concentration of the acetone immediately after mixing (in m). give your answer to 3 sig figs)
Answers
concentration of the acetone immediately after mixing
We are given:
- Molarity of Acetone\($=4.0 \mathrm{M}$,\)
- Volume of Acetone\($=11 \mathrm{~mL}=0.011 \mathrm{~L}$,\)
- Volume of\($B r_2=16 \mathrm{~mL}$,\)
- Volume of \($\mathrm{HCl}=11 \mathrm{~mL}$\), and
- Volume of Water\($=16 \mathrm{~mL}$.\)
therefore Total Volume of Solution\($=(11+16+11+16) m L=54 m L=0.054 L$\)and
Total No. of moles \(\mathrm{M} \times 0.011 \mathrm{~L}$$=0.044 \mathrm{~mol}$\)of Acetone = Molarity of Acetone \($\times$\) Volume of Acetone i =4.0
\($\therefore$ Molarity of Acetone $=\frac{\text { No. of moles of Acetone }}{\text { Volume of solution in liter }}$\)
\($=\frac{0.044 \mathrm{~mol}}{0.054 \mathrm{~L}}$$=0.815 M$Hence, Concentration of Acetone (in Molarity) is $0.815 \mathrm{M}$.\)
To learn more about Acetone visit:
https://brainly.com/question/13334667
#SPJ4
the exchange capacity of an ion-exchange resin is defined as the number of moles of charged sites per gram of dry resin. describe how you would measure the exchange capacity of an anion-exchange resin by using standard naoh, standard hcl, or any other reagent you wish.
Answers
The exchange capacity of an anion-exchange resin can be measured using a titration method. First, a known weight of the resin is weighed out and suspended in a buffered solution of a known pH.
The resin is then titrated with a standard solution of either sodium hydroxide (NaOH) or hydrochloric acid (HCl) until the pH of the solution reaches a predetermined endpoint. The amount of titrant added during the titration is then used to calculate the moles of charged sites per gram of dry resin.
To calculate the exchange capacity, the following equation can be used:
Exchange Capacity (mol/g dry resin) = (mol titrant added) / (g dry resin)
The mol titrant added is determined from the titration curve, which is constructed by measuring the pH of the solution versus the volume of titrant added. The endpoint of the titration is typically assumed when the pH of the solution reaches a predetermined value.
To ensure accuracy, the titration should be repeated at least three times and the average of the results should be taken. This will ensure that the exchange capacity measured is an accurate representation of the resin.
To know more about resin, click below:
https://brainly.com/question/28487831
#SPJ4
One-half gram of solid calcium sulfate, CaSO_4 (s), is added to 1.0 L of pure water. Immediately, the solid begins to dissolve according to the following reaction: CaSO_4(s) reversible Ca^2+ + SO_4^2- What is the concentration of dissolved sulfate in the water once equilibrium is achieved
Answers
The solubility equilibrium for calcium sulfate is given by:
CaSO4(s) ⇌ Ca2+(aq) + SO42-(aq)
At equilibrium, the rate of dissolution of CaSO4(s) is equal to the rate of precipitation of CaSO4(s), and the concentrations of Ca2+(aq) and SO42-(aq) remain constant.
Let x be the concentration of SO42-(aq) at equilibrium. At the beginning of the reaction, before any dissolution has occurred, the concentration of SO42-(aq) is zero. As the solid dissolves, it produces Ca2+(aq) and SO42-(aq) ions in a 1:1 mole ratio. Therefore, at equilibrium, the concentration of Ca2+(aq) is also equal to x.
The solubility product expression for calcium sulfate is:
Ksp = [Ca2+][SO42-]
At equilibrium, we can substitute x for [Ca2+] and [SO42-] in this expression:
Ksp = x^2
The value of Ksp for calcium sulfate at 25°C is 4.93 × 10^-5. Therefore:
4.93 × 10^-5 = x^2
Taking the square root of both sides:
x = 7.02 × 10^-3 M
Therefore, the concentration of dissolved sulfate in the water once equilibrium is achieved is 7.02 × 10^-3 M.
Visit here to learn more about equilibrium brainly.com/question/30807709
#SPJ11
Need help with question 1.) I got something wrong but i don’t know hurry ASAP

Answers
Vinegar is an heterogeneous mixture
Explanation:
Which of the following metals are suitable for use as sacrificial anodes to protect against corrosion of underground iron pipes? If any are not suitable, explain why:
(a) Aluminum, (b) , (c) , (d) , (e) , (f) , (g)
Answers
The suitable metals for use as sacrificial anodes to protect against corrosion of underground iron pipes are magnesium, zinc, and aluminum.
These metals have a higher electrochemical potential than iron, which causes them to corrode instead of the iron pipe. This sacrificial corrosion helps protect the iron pipe from further damage. Other metals like copper, silver, and gold are not suitable as sacrificial anodes because they have a lower electrochemical potential than iron, meaning they will not corrode in preference to the iron pipe.
to know more about metals intake pls visit:
https://brainly.com/question/25749576
#SPJ11
Rosa was looking for patterns to help predict the products of chemical reactions. She recorded three similar decomposition reactions in the table. What products should she record in the last row of the table? 2licl + 3o2 3licl + 2o2 2lio + 3cl2 3lio+ 2cl2.
Answers
The products Rosa recorded in the last row of the table should be: 2LiCl + 3O₂.
The type of reaction which Rosa did is a decomposition reaction which involves one compound that yields to more than one (or usually two) product. To determine the product, we can deduce that it has to contain elements of Li, Cl and O₂. So, from the options, the answer is 2LiCl + 3O₂.
What is a decomposition reaction?A decomposition reaction can be described as a chemical reaction in which one reactant breaks down into two or more products. Generally, decomposition reactions need energy input.
Learn more about decomposition reaction at: https://brainly.com/question/16987748
#SPJ4

What is the hydrogen ion concentration of a strong acid which has a pH of 6.3
Answers
Answer:
5.0 x 10^-7
Explanation:
[H+]=10^-6.3
How many elements in a periodic table?
Answers
How much hear must be obsorberd by 375.0 grams of water to raise its temperature from 10.0c to 35.0c? The specific heat capacity of water is 4.186 J/gC.
HELP DUE TOMORROW!!! ASAP

Answers
Answer:
The heat absorbed by water is 39243.75 J.
Explanation:
Given data:
Mass of water = 375.0 g
Heat absorbed by water= ?
Initial temperature = 10.0°C
Final temperature = 35.0 °C
The specific heat capacity of water = 4.186 j/g.°C
Solution:
Specific heat capacity:
It is the amount of heat required to raise the temperature of one gram of substance by one degree.
Formula:
Q = m.c. ΔT
Q = amount of heat absorbed or released
m = mass of given substance
c = specific heat capacity of substance
ΔT = change in temperature
ΔT = T2 - T1
ΔT = 35°C - 10°C
ΔT = 25°C
Q = m.c. ΔT
Q = 375.0 g× 4.186 j/g °C × 25°C
Q = 39243.75 J
The heat absorbed by water is 39243.75 J.
when nh4no3 dissolves in water, the temperature of the solution decreases. what describes the enthalpy and entropy changes of the system and which change drives the process? (a) delta h
Answers
When \(NH_4NO_3\) dissolves in water, the enthalpy of the system decreases, and the entropy of the system increases. The increase in entropy is the driving force of the process, causing the solute to dissolve in the solvent. Option A is the correct answer.
When \(NH_4NO_3\) dissolves in water, it undergoes an endothermic process in which heat is absorbed from the surroundings, causing the temperature of the solution to decrease. This suggests that the dissolution process is driven by an increase in the system's entropy, rather than by a decrease in enthalpy.
The enthalpy change of the system can be determined by measuring the heat of the solution, which is the amount of heat absorbed or released when a solute dissolves in a solvent. In the case of \(NH_4NO_3\), the heat of the solution is positive, indicating an endothermic process in which heat is absorbed. This means that the enthalpy of the system decreases when \(NH_4NO_3\) dissolves in water.
The entropy change of the system can be determined by calculating the difference in the entropy of the solution and the sum of the entropies of the separate components (the solute and the solvent) before mixing. In the case of \(NH_4NO_3\) dissolving in water, the increase in the disorder of the system is driven by the release of ammonium ions and nitrate ions into the solvent. The disorder of the system increases because the ions become more dispersed and move around freely in the solution. This increase in entropy is the driving force of the process.
To learn more about enthalpy
https://brainly.com/question/16720480
#SPJ4
Complete question:
Which of the following describes the enthalpy and entropy changes of the system when \(NH_4NO_3\) dissolves in water, and which change drives the process?
a) Enthalpy decreases and entropy increases; entropy change drives the process
b) Enthalpy increases and entropy decreases; enthalpy change drives the process
c) Enthalpy decreases and entropy decreases; enthalpy change drives the process
d) Enthalpy increases and entropy increases; entropy change drives the process
At room temperature I2(s) is a molecular solid. Which of the following provides a characteristic of I2(s) with a correct explanation?O It has a high melting point because it has weak intermolecular forces.
O It is hard because it forms a threedimensional covalent network.
O It is not a good conductor of electricity because its valence electrons are localized in bonding and nonbonding pairs.
O It is very soluble in water because its molecules are polar.
Answers
Due of the weak intermolecular interactions, it has a high melting point.
A force that attracts the protons or positive parts of one molecule to the electrons or negative parts of another molecule is known as an intermolecular force. A substance's various physical and chemical properties are influenced by this force. The strength of an object's intermolecular forces determines its boiling point; the higher the intermolecular forces, the higher the boiling point.
We can compare the intermolecular forces between different substances by comparing their boiling points. This is so that these intermolecular interactions can be broken and the liquid can be transformed into vapour using the heat that the substance absorbs at its boiling point.
To know more about intermolecular forces
https://brainly.com/question/9007693
#SPJ4
which is more flammable hexane or potassium sulfate
Answers
Answer:
Between the two compounds, hexane and potassium sulphate, hexane is the more flammable compound.
Explanation:
Among hexane and potassium sulfate, hexane is more flammable.
What is Flammability?Flammability may be defined as a type of physical property of an element or compound which determines the ability to support combustion. Due to this, it is also known as Combustibility.
This property defines the capability of any chemical element in order to ignite and continue it a very long time after coming or exposing it to direct contact with a flame or any source of ignition.
The characteristic of flammability relies on the volatility of the compound. This means that the higher the volatility of the compound greater or high the flammability.
So, when we compare hexane or potassium sulfate on the basis of volatility, hexane is more volatile and thus possesses high flammability.
Therefore, among hexane and potassium sulfate, hexane is more flammable.
To learn more about Flammability of chemical compounds, refer to the link:
https://brainly.com/question/14214497
#SPJ2
the decomposition of hydrogen peroxide is catalyzed by iodide ion what happen to catalyst concentration
Answers
The decomposition of hydrogen peroxide into water and oxygen is a slow process, but it can be catalyzed by iodide ion. The iodide ion acts as a catalyst by lowering the activation energy required for the reaction to occur.
During the reaction, the iodide ion is oxidized to form iodine, which then reacts with hydrogen peroxide to form water and oxygen. The iodine can then react with more hydrogen peroxide to continue the reaction.
The concentration of the catalyst, iodide ion, affects the rate of the reaction. An increase in the concentration of the iodide ion will increase the rate of the reaction, as there will be more catalyst available to facilitate the reaction. Conversely, a decrease in the concentration of the iodide ion will slow down the rate of the reaction.
However, once the reaction has finished, the concentration of the catalyst will remain the same. This is because the catalyst is not consumed in the reaction and can be used again in subsequent reactions. Therefore, the concentration of the catalyst will remain constant as long as there is enough iodide ion present to catalyze the reaction.
To learn about decomposition
https://brainly.com/question/8009068
#SPJ4
explain how you could use ir spectroscopy to differentiate between compounds f and g. (b) explain how you could use ir spectroscopy to differentiate between compounds d and e. (c) if you wanted to distinguish between compounds b and f, would it be more suitable to use ir spectroscopy or mass spectrometry? explain. (d) would mass spectrometry be helpful for distinguishing between compounds a and d? explain.
Answers
Since these two substances are both alcohols, IR spectroscopy cannot be used to separate them.
These two substances may be distinguished from one another using mass spectrometry because they have various molecular weights.The IR spectroscopy theory is based on the idea that molecules have a tendency to absorb particular light frequencies that are unique to the corresponding structure of the molecules.The electromagnetic spectrum's infrared section, which includes light with a longer wavelength and lower frequency than visible light, is the subject of infrared spectroscopy (IR spectroscopy).
Learn more about IR spectroscopy here:
https://brainly.com/question/29441785
#SPJ4
when 70.0 g of li3n(s) reacts with excess h2(g), 8.0 g of linh2 (s) is produced. the percent yield is closest to
Answers
when 70.0 g of li3n(s) reacts with excess h2(g), 8.0 g of linh2 (s) is produced. The percent yield is closest to 17%
How to calculate the percentage yield
The reaction between Li3N(s) and LiH(s) is given as:
Li3N+3H2 = 3LiH + NH3
Calculate the moles of Li3N
Moles = Mass/Molar mass
Moles = 70/35
Moles = 2 moles
From the reaction you can see that 1 mole of Li3N produce 3 moles of LiH, hence the moles of LiH is 6 moles
Determine the mass of LiH (theoretical yield)
Mass of LiH = 6 * 7.95
Mass of LiH = 47.7g
%yield = actual/theoretical * 100
%yield = 8/47.7 * 100
%yiels = 16.77%
Hence the percent yield is closest to 17%
Learn more on percent yield here:
https://brainly.com/question/12704041
#SPJ4
how to tell how many valence electrons an atom has?
Answers
Answer: Count only the electrons in the highest shell s and p orbitals when determining valence.
Explanation: I'm glad you asked this question. It is often not well explained. The term valence electrons is assigned to only the electrons in an element's highest energy level. These reside only in the s and p orbitals, and not the d or f, as I'll explain later. The s orbital can hold 2 electrons and the p can hold 6. Potassium, K, has an s orbital in its highest energy shell, 4. It contains only 1 electron, so it has a valency of 1.
Calcium, Ca, has 2 in its highest energy level: 4s^2, so it has a valency of 2. Moving to the right, the element scandium, Sc, add another electron, but it goes into the 3d orbital. 3d is in the 3rd energy shell, so it is not counted as a valence electron. Only after we move further right, to gallium, Ga, do we start adding electrons to the 4th energy level again - the 4p orbitals can accept up to 6 electrons. Ga has 3 valence electrons - 2 in the 4s and 1 in the 4p. Oxygen has 2 in the 2s and 4 in the 4p orbitals, for a total of 6. It is close to having a comple outer shell (2 in the 2s and 6 in the 2p). Just 2 more electrons would fill both the 2s and 2p orbitals for a total of 8 valence electrons, a stable configuration (the same configuration as thje stable gas Neon).
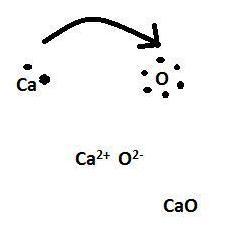
the correct lewis dot structure for CaO?
Answers
Answer:
Here you go.
Explanation:
Hopefully it is not confusing.
What is the disaccharide composed of glucose and fructose?
Answers
The disaccharide are composed of the glucose and the fructose is the sucrose.
The disaccharide composed of the glucose and the fructose is sucrose. The sucrose is commonly called as the table sugar. The glucose and the fructose both are the monosaccharides and if they combined together , they form the disaccharide sugar.
The sucrose is produced as naturally in the plants and is then extracted and will processed to form the sugar and this is used in the cooking as the sweetener. The sucrose is the one of the most abundant and it consists of the molecule of the α-glucose and the β-fructose linked together.
To learn more about glucose here
https://brainly.com/question/28330296
#SPJ4
How does hydrogen bonding take place?
Answers
Answer:results from the attractive force between a hydrogen atom covalently bonded to a electronegative atom
Explanation:
What energy transfer is occurring when a battery-powered toy rolls across the floor? a Stored mechanical energy is converted to thermal energy. b Stored mechanical energy is converted to mechanical energy. c Stored chemical energy is converted to thermal energy. d Stored chemical energy is converted to mechanical energy.
Answers
Answer:
The correct option is;
d Stored chemical energy is converted to mechanical energy
Explanation:
Energy is stored in a battery in the form of electric potential energy. The chemical in a battery are such that they have an electric potential between them when charged and when the terminals of the battery are connected to an external circuit, the potential difference between the compounds at the poles causes electric current to flow from one pole, across the circuit and enter back into the battery through the other pole
The energy of the moving electrons can then be used to do mechanical work such as causing the mechanism of a coil in a magnetic field to rotate. The rotation can then be used to turn the wheels of the toy using gears.
Place the following substances in order of increasing volatility: CH4, CBr4, CH2Cl2, CH3Cl, CHBr3, and CH2Br2. (b) How do the boiling points vary through this series? (c) Explain your answer to part (b) in terms of intermolec- ular forces.
Answers
The substances in order of increasing volatility are: CBr4, CHBr3, CH2Br2, CH3Cl, CH2Cl2, CH4.
The boiling points increase as the substances become heavier, with CH4 having the lowest boiling point and CBr4 having the highest boiling point.
This trend can be explained by intermolecular forces. The substances with stronger intermolecular forces will have higher boiling points. In this series, the substances with higher molecular weights have more electrons, which leads to stronger London dispersion forces.
Additionally, the substances with more polar bonds, such as CH3Cl and CH2Cl2, also have stronger dipole-dipole forces. Overall, the combination of these intermolecular forces determines the boiling points of the substances in this series.
To know more about intermolecular forces:
https://brainly.com/question/9328418
#SPJ11
Please help me I have a lot of points

Answers
Answer: Five moles of CH4 would require ten moles of O2.
Explanation: Start with a balanced equation. You can see from the balanced equation that one mole of CH4 reacts completely with two moles of O2. So five moles of CH4 would require ten moles of O2.
If, per se, juice is denser than water how do they mix?
Answers
Conduction can only occur between two objects when – both objects are exactly the same temperature. Both objects are exactly the same temperature. One of the objects is made of a metal. One of the objects is made of a metal. Both objects are in physical contact with each other. Both objects are in physical contact with each other. One object is less dense than the other. One object is less dense than the other.
Answers
Answer:
Both objects are in physical contact with each other
Explanation:
Conduction refers to the flow of heat from one object to another when the both objects are in contact with each other.
Hence, before heat can flow from one object to another, the two objects must be in contact with each other. This is a key requirement before conduction can take place.
How many Liters are in 3 grams of O2? *
Answers
if you gently shake a separatory funnel containing a carboxylic acid with ethyl acetate and aq nahco3, the organic acid will be found where? (multiple answers possible)
Answers
The organic compound, carboxylic acid will be found in ethyl acetate.
What are organic compounds?Organic compounds are those compounds that are obtained naturally from living matter.
Organic compounds are mostly composed of carbon, hydrogen, and oxygen.
Organic compounds are generally covalent compounds. Hence, they are usually non-polar compounds.
Based on the principle of like dissolves like, organic compounds are usually soluble in non-polar organic solvents and insoluble in polar inorganic solvents like water.
Carboxylic acid is an organic compound.
Ethyl acetate is an organic compound.
Aqueous NaHCO₃ is an inorganic compound.
Hence, carboxylic acid will dissolve in Ethyl acetate.
Learn more about organic compounds at: https://brainly.com/question/6279332
#SPJ1
PDB CODE: 1MRY SEQUENCE POSITION: 229 AMINO ACID MUTATED TO: ARG In the PDB protein, you were given the sequence position of a particular amino acid that is mutated to another amino acid. Draw the structure of the two amino acids. Describe why this position in your protein is important and outline the effects of the mutation will have on the 3-D structure and the function of your protein.
Answers
The mutated amino acid at position 229 is arginine (Arg), and its substitution can potentially disrupt the 3D structure and alter the function of the protein due to changes in side chain properties and interactions.
Structure of the Amino Acids:
The wild-type amino acid at position 229 is not specified, so the structure cannot be provided.
The mutated amino acid is arginine (Arg), which has a side chain containing a positively charged guanidinium group.
Importance of the Position:
The specific position 229 in the protein sequence may be functionally significant, such as being involved in protein-protein interactions, binding sites, catalytic activity, or structural stability.
Without detailed knowledge of the protein, its function, and its structural context, it is difficult to determine the exact importance of this specific position.
Effects of the Mutation on Structure and Function:
The substitution of an amino acid at position 229 from the original to arginine can have various effects on protein structure and function.
Arginine's larger and positively charged side chain may introduce steric clashes or alter electrostatic interactions within the protein structure.
The mutation can potentially disrupt local or global protein folding, stability, or conformational changes, affecting its overall 3D structure.
The functional consequences of the mutation depend on the specific role of the amino acid at position 229, which can include changes in protein-protein interactions, enzymatic activity, substrate binding, or signal transduction pathways.
It is crucial to analyze the protein's structural context, available experimental data, and computational modeling techniques to gain a more accurate understanding of the specific effects of the mutation on the protein's structure and function in the given context.
Learn more about Amino Acids from the given link:
https://brainly.com/question/31872499
#SPJ11
Part B: The area of a rectangle is (4x2 − 9y2) square units. Determine the dimensions of the rectangle by factoring the area expression completely. Show your work
Answers
The dimensions of the rectangle are (2x + 3y) and (2x - 3y). The length is (2x + 3y) units, and the width is (2x - 3y) units.
To determine the dimensions of the rectangle with an area of (4x^2 - 9y^2) square units, we need to factor the area expression completely.
First, let's observe that the given expression is a difference of squares, which can be factored using the identity: a^2 - b^2 = (a + b)(a - b).
In our case, we have (4x^2 - 9y^2), which can be written as (2x)^2 - (3y)^2. Now we can apply the difference of squares formula:
(2x)^2 - (3y)^2 = (2x + 3y)(2x - 3y)
The factored form of the area expression is (2x + 3y)(2x - 3y). From this, we can identify the dimensions of the rectangle.
The length of the rectangle is represented by the sum of the factors, (2x + 3y), and the width is represented by the difference of the factors, (2x - 3y).
By factoring the area expression completely, we can see how it relates to the dimensions of the rectangle. It helps us understand the factors that contribute to the area and provides a clearer representation of the rectangle's dimensions.
To know more about dimensions of the rectangle, please click on:
https://brainly.com/question/31677552
#SPJ11
issued this? watch kcv: atomic theory; read section 2.3. you can click on the review link to access the section in your etext. carbon and oxygen form both carbon monoxide and carbon dioxide. when samples of these are decomposed, the carbon monoxide produces 3.36 g of oxygen and 2.52 g of carbon, while the carbon dioxide produces 9.92 g of oxygen and 3.72 g of carbon.
Answers
The atomic ratio of carbon to oxygen in carbon monoxide (CO) is 1:1, and the atomic ratio of carbon to oxygen in carbon dioxide (CO₂) is 2:1.
Firstly, we can analyze the decomposition of carbon monoxide (CO) and carbon dioxide (CO₂) to determine the atomic ratios involved.
Let's denote the atomic ratio of carbon to oxygen in carbon monoxide as x, and the atomic ratio of carbon to oxygen in carbon dioxide as y.
According to the given data;
Decomposition of carbon monoxide (CO);
Oxygen produced = 3.36 g
Carbon produced = 2.52 g
We know that the atomic mass of carbon is 12 g/mol, and the atomic mass of oxygen is 16 g/mol. Using these values, we can calculate the number of moles for each element;
Number of moles of oxygen = mass / atomic mass = 3.36 g / 16 g/mol = 0.21 mol
Number of moles of carbon = mass / atomic mass = 2.52 g / 12 g/mol = 0.21 mol
Since the atomic ratio of carbon to oxygen in carbon monoxide is x, we can write the following equation;
0.21 mol C / (0.21 mol O) = x
Simplifying the equation, we have;
x = 1
Therefore, the atomic ratio of carbon to oxygen in carbon monoxide is 1:1.
Decomposition of carbon dioxide (CO₂);
Oxygen produced = 9.92 g
Carbon produced = 3.72 g
Following the same calculations as before;
Number of moles of oxygen = mass / atomic mass = 9.92 g / 16 g/mol = 0.62 mol
Number of moles of carbon = mass / atomic mass = 3.72 g / 12 g/mol = 0.31 mol
Since the atomic ratio of carbon to oxygen in carbon dioxide is y, we can write the following equation;
0.31 mol C / (0.62 mol O) = y
Simplifying the equation, we have;
y = 0.5
Therefore, the atomic ratio of carbon to oxygen in carbon dioxide is 1:0.5, which can be simplified to 2:1.
To know more about decomposition here
https://brainly.com/question/20418092
#SPJ4
--The given question is incomplete, the complete question is
"Missed this? watch kcv: atomic theory; read section 2.3. you can click on the review link to access the section in your text. carbon and oxygen form both carbon monoxide and carbon dioxide. when samples of these are decomposed, the carbon monoxide produces 3.36 g of oxygen and 2.52 g of carbon, while the carbon dioxide produces 9.92 g of oxygen and 3.72 g of carbon. Calculate the atomic ratio of carbon to oxygen in carbon monoxide, and carbon dioxide."--