Please help and hurry! I'll give brainliest
Physical Models Activity
You have explored some interesting, informative, and amusing examples of models. Now it's time to get creative and make your own model. Here is the requirement checklist for your model:
✔ Model types can include drawings, diagrams, physical models, virtual simulations, or videos.
✔ Models must be created by you, not something selected from an online or outside source.
✔ Submit a presentation, picture, video, or screenshot of your model.
✔ Submit a one-paragraph summary describing the topic you chose, your model, what it represents, how you made it, and the specific science involved. It is important that you are using science terminology and are accurate.
Now that you know how to create and submit your model, you will need to choose a topic for your model. Choose one of the three topics listed below. Select each topic for an overview.
Conservation of Mass
Atomic Theory
Thermal Energy
Here's a student example to help guide you. This is one of many ways to model the topic options. If you have an idea but are not sure, you can contact your instructor for assistance.
To learn more about how you will be graded on this assignment, review the grading rubric.
Answers
Answer:
hi this it for the Instructions: In this engineering lab, you will build and test a device that releases and absorbs
thermal energy in order to reach a goal. You will need to repeat tests of your device to make sure it
does not need to be redesigned or improved. Record your observations and test measurements in
the lab report below. You will submit your completed report. yea that thing the answer is in the file
Explanation:
sorry for telling you on this :( but i hope this helps
Related Questions
A fixed mass of oxygen gas occupies 300cm cube at 0 degree centigrade. what volume would the gas occupy at 15 degree centigrade
Answers
Answer:
Volume occupied by oxygen gas at 15 degree centigrade is equal to \(316.5\) centimeter cube
Explanation:
Assuming Pressure is constant.
\(\frac{V_1}{T_1} = \frac{V_2}{T_2}\)
where T1 and T2 are temperature in Kelvin
Substituting the give values we get-
\(\frac{300}{273} = \frac{V_2}{288}\)
\(V_2 = \frac{288*300}{273} \\V_2 = 316.5\)
Volume occupied by oxygen gas at 15 degree centigrade is equal to \(316.5\) centimeter cube
When the equation
Cu + AgNO3 → Cu(NO3)2 + Ag
is balanced, the coefficient of copper (II) nitrate is
A. 0.
B. 3.
C. 1
Answers
An equation which follow the law of conservation of mass is called the balanced equation. The coefficient of copper (II) nitrate is 1 .The correct option is C.
What is a balanced equation?A chemical equation in which the number of atoms of reactants and products on both sides of the equation are equal is defined as the balanced chemical equation.
The coefficients are the numbers which are used to balance the chemical equation. They are added in front of the formulas. According to law of conservation of matter, mass can neither be formed nor be destroyed.
The balanced equation for the given reaction is:
Cu (s) + 2AgNO₃ (aq) → Cu (NO₃)₂ (aq) + 2Ag (s)
Here the coefficient of Cu (NO₃)₂ is 1. The number of 'Cu', 'Ag', 'N' and 'O' atoms are equal. So the given equation is balanced. The coefficient of AgNO₃ is 2, and 'Ag' is 2.
Thus the correct option is C.
To know more about balanced equation, visit;
https://brainly.com/question/29769009
#SPJ2
what are the subatomic particles by which atom made of?
Answers
Answer:
The three main subatomic particles that form an atom are protons, neutrons, and electrons.
Explanation:
Answer:
The three main subatomic particles that form an atom are protons, neutrons, and electron
What can you determine about an element if you only know its group number on the periodic table?
A. The total number of protons and neutrons
B. The number of protons
C. The total number of electrons
D. The number of valance elctrons
Answers
Answer:
c
Explanation:
Plz help asap What can you conclude about the iron(ii) and iron(iii) ions?

Answers
Answer:
The chemistry of iron is dominated by the +2 and +3 oxidation states i.e. iron(II) and iron(III) complexes e.g. Fe2+ and Fe3+ complex ions with selected ligands, usually of an octahedral shape, a few tetrahedral iron(III) complexes are mentioned too. The reactions of the aqueous ions iron(II) and iron(III) with ammonia, sodium hydroxide and sodium carbonate are described and explained as are complexes of iron(III) with the chloride ion and cyanide ion.
principal oxidation states of iron, redox reactions of iron, ligand substitution displacement reactions of iron, balanced equations of iron chemistry, formula of iron complex ions, shapes colours of iron complexes, formula of compoundsExplanation:
I know I made a few errors but I don’t know which ones please help me

Answers
Answer:
ooooooooooooooooooooooooooooooooooooooh
A boy swings a rubber ball attached to a string over his head in a horizontal, circular path. The piece of string is 1.15 m long and the ball makes 137 complete turns each minute. What is the tangential velocity of the ball
Answers
Answer:
v = 16.49 m/s
Explanation:
Given that,
Length of the string, l = 1.15 m
The ball makes 137 complete turns each minute.
We know that, 1 turn = 6.28 rad
137 turns = 860.79 rad
1 min = 60 s
\(\omega=\dfrac{860.79\ rad}{60\ s}\\\\=14.34\ rad/s\)
We need to find the tangential velocity of the ball. It can be given by
\(v=r\omega\\\\=1.15\times 14.34\\\\v=16.49\ m/s\)
So, the tangential velocity of the ball is 16.49 m/s.
If a piece of copper metal is placed in calcium chloride solution. What will you observe? Give reason for your answer.
Answers
Answer:
Explanation:
Reduction potential of Copper metal is .34 V and reduction potential of Calcium is - 2.87 V . So copper will not be able to reduce Calcium chloride to calcium . Hence no reaction will take place when a piece of copper metal is placed in calcium chloride solution .
The mixture in the large beaker still looked clear like water, but when the students, one at a time, carefully touched the outside of the large beaker, it felt warm to the touch.
Why did the large beaker most likely feel warm?

Answers
Answer:
A chemical reaction produced a new substance.
Explanation:
I just answered this on the study island quiz.
Answer:
A chemical reaction produced a new substance or "A"
Explanation:
Cu2(s)+O2(g)=Cu2O(g)+SO2(g)
please help urgent will give brainiest
Answers
Answer:
2 Cu2S + 3 O2 = 2 Cu2O + 2 SO2
Explanation:
2 Cu2S + 3 O2 = 2 Cu2O + 2 SO2
What are the 4 types of characterization?.
Answers
Answer:
There are actually five, and there's an easy way to remember them.
Physical Description
Action
Inner thoughts
Reactions
Speech
P.A.I.R.S
This will basically help you with any story you come up with
Question 3 please help :)

Answers
Explanation:
Removing B from the system
- Decreases the rate of the reaction. Backward reaction (formation of reactants) is favoured.
Crushing A into a powder
- Increases the rate of reaction. This is because of the increased surface area of A.
Warming the system
- Increases the rate of the reaction. Temperature is proportional to rate of reaction.
Adding more A to the system
- Increases the rate of reaction. Forward reaction (formation of products) is favoured.
Putting the system into an ice bath
- Decreases the rate of reaction. Temperature is proportional to rate of reaction.
Decreasing the pressure of the system
- Decreases the rate of the reaction.
calculate mass of gum you need to chew to chew one mole of sugar
Answers
The mass of gum you need to chew to chew one mole of sugar is approximately 5.68 × 10-22 grams.
To calculate the mass of gum that you need to chew to chew one mole of sugar, you need to use the following steps:
Step 1: Find the molar mass of the sugar
The molar mass of sugar is 342.3 g/mol.
Step 2: Convert molar mass to gramsTo convert the molar mass to grams, you need to divide it by Avogadro's number (6.022 × 1023), which gives you 5.68 × 10-22 grams of sugar.
Step 3: Find the mass of gum needed since it is not provided how much sugar is present in a single piece of gum, we can assume that it is negligible.
Therefore, the mass of gum needed to chew one mole of sugar is approximately 5.68 × 10-22 grams.
Hence, the mass of gum you need to chew to chew one mole of sugar is approximately 5.68 × 10-22 grams.
To know more about mole refer to:
https://brainly.com/question/29367909
#SPJ11
On the basis of electronegativity differences between atoms, which of the following scientific claims is the most accurate regarding the bonding in Mg(NO3)2(8) ?
A) There is polar covalent bonding between Mg atoms and N atoms.
B) There is polar covalent bonding between Mg atoms and O atoms.
C) There is ionic bonding between N5+ ions and O2- ions.
D) There is ionic bonding between Mg2+ ions and NO3- ions.
Answers
There is ionic bonding between Mg²⁺ ions and NO₃⁻ ions. A Further explanation is below.
Ionic bonding seems to be a form of interaction wherein the cationic as well as unpaired electrons are powerfully electrostatically connected.
Throughout the general view, the ionic bonding occurs between someone metal/nonmetal owing to their large electro(-) discrepancy, while bond formation occurs between nonmetals due to the relatively low electro(-) gap.Even though there are metals as well as non-metals, the relative electronegativity differential between (Mg²⁺) and (NO₃⁻) however is large.Thus the above response i.e., option D) is correct.
Learn more about electronegativity here:
https://brainly.com/question/24431979

Which molecule contains the most easily broken carbon-carbon bond? H_2C=CH_2 OF_2C=CF_2 H_3C-CH_3 Triple bond in N2
Answers
H₂C=CH₂ contains the most easily broken carbon-carbon bond.
Which molecule among H₂C=CH₂, OF₂C=CF₂, H₃C-CH₃, and N₂ has the weakest carbon-carbon bond?Among the given options, the molecule with the most easily broken carbon-carbon bond is H₂C=CH₂.
In this molecule, the carbon-carbon bond is a double bond, consisting of one sigma bond and one pi bond. Pi bonds are generally weaker than sigma bonds due to their overlapping electron density being spread out above and below the bond axis. As a result, pi bonds are more susceptible to breakage compared to sigma bonds.
On the other hand, in OF₂C=CF₂, the carbon-carbon bond is also a double bond, but the presence of fluorine atoms enhances the bond strength due to their high electronegativity.
In H₃C-CH₃, the carbon-carbon bond is a single bond, which is stronger than a double bond and less prone to breaking.
The triple bond in N₂ is the strongest among the given options, requiring a significant amount of energy to break.
Therefore, H₂C=CH₂ contains the most easily broken carbon-carbon bond.
Learn more about H₂C=CH₂
brainly.com/question/30645266
#SPJ11
_________ is the attractive force between all objects. A. Gravity B. Orbit C. Nuclear force D. Prettiness
Answers
Gravity is the attractive force between all objects. The correct answer is A.
Gravity is the fundamental force of attraction that exists between all objects with mass. It is responsible for the formation and behavior of planets, stars, galaxies, and the entire universe. The force of gravity depends on the masses of the objects and the distance between them, and it acts in all directions. Gravity is what keeps us grounded on Earth, and it is also responsible for the motion of objects in space. The laws of gravity were first described by Sir Isaac Newton in the 17th century and later refined by Albert Einstein's theory of general relativity in the 20th century. The correct answer is option A.
To know more about Gravity, here
brainly.com/question/31321801
#SPJ1
Which of the following acids has the strongest conjugate base?
Question options:
a. HClO
b.HClO_4
c.HClO_2
d.HClO_3
e.HCl
Answers
Conjugate bases are defined as the molecules or ions that remain after acids donate a proton. Conjugate base strength is determined by the strength of the parent acid. As a result, strong acids generate weak conjugate bases, while weak acids generate strong conjugate bases.
The correct option is b. HClO_4.HClO4 is the strongest acid since it has the highest Ka. Because the conjugate base of HClO4 is ClO4, which is quite stable and can handle negative charges better than the others, it is the strongest conjugate base. HClO4 is the strongest acid among the options provided because it has the highest Ka. The conjugate base of HClO4 is ClO4, which is a very stable base because it can manage the negative charge better than any other options provided.
Learn more about Conjugate bases here ;
https://brainly.com/question/30086613
#SPJ11
Chemistry Solutions and Reaction Rates

Answers
Answer:
D. decreasing the surface area of Zn
Explanation:
Because zinc is a reactant in the reaction, decreasing its surface area would decrease the production rate of ZnCl₂ - there are fewer particles exposed to the hydrochloric acid so there is a smaller chance of particles colliding which means there are fewer successful collisions per second.
Hope this helps!
A uniform, solid disk with a mass of 24. 3 kg and a radius of 0. 314 m is free to rotate about a frictionless axle. Forces of 90 n and 125 n are applied to the disk in the same horizontal direction, but one force is applied to the top and the other is applied to the bottom. What is the magnitude of the angular acceleration of the disk?.
Answers
The magnitude of the angular acceleration of the disk is -203.9 rad/s^2 (negative sign is indicating the direction of angular acceleration is opposite to the direction of applied forces).
The magnitude of the angular acceleration of the disk can be determined by using the equation for torque:
τ = Iα
where τ is the torque, I is the moment of inertia, and α is the angular acceleration.
The moment of inertia of a solid disk is given by the equation:
I = (1/2) * m * r^2
where m is the mass and r is the radius of the disk.
So we can substitute the values of mass and radius in the equation of the moment of inertia:
I = (1/2) * 24.3 kg * (0.314 m)^2 = 0.03898 kg*m^2
Now we can calculate the torque caused by the forces of 90 n and 125 n. These forces are applied in opposite direction so the net torque caused by them is:
τ = 90 N * 0.314 m - 125 N * 0.314 m = -7.922 Nm
So we can now calculate the angular acceleration using the torque equation:
-7.922 Nm = 0.03898 kgm^2 * α
α = -7.922 Nm / 0.03898 kgm^2 = -203.9 rad/s^2
Therefore, The magnitude of the angular acceleration of the disk is -203.9 rad/s^2 (negative sign is indicating the direction of angular acceleration is opposite to the direction of applied forces).
Learn more about angular acceleration here:
https://brainly.com/question/29428475
#SPJ4
clerice midter
The diagram below is the Bohr model of an atom.
Which best describes this atom?
OA. It has 6 electrons.
OB. It has a positive charge.
O c. It has 6 valence electrons.
OD.
has a full outermost energy level.
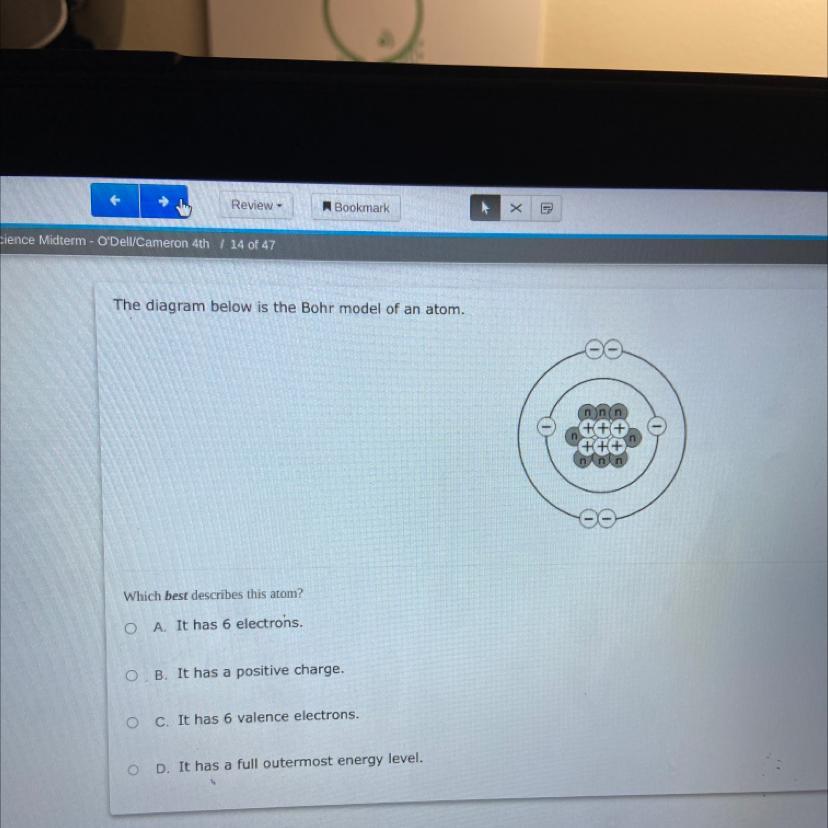
Answers
The correct option is (A) - This Bohr Model of atom describes that there are a total of 6 electrons in the given figure.
What is Bohr Model of atom?The electrons are positioned in circular orbitals at particular distances from the central nucleus in the Bohr model of the atom. These orbits create electron shells or energy levels, which allow us to see how many electrons are present in each shell. The number and the letter "n" are used to identify these energy levels. The first energy level nearest to the nucleus, for instance, is represented by the 1n shell. Normally, an electron resides in the shell with the lowest energy, which is the one closest to the nucleus. A photon of light's energy can raise it to a higher energy shell, but this is an unstable position, and the electron quickly returns to the ground state.
Learn more about atom here:
https://brainly.com/question/30898688
#SPJ1
What do you think happens to the bonds between atoms when substances melt?
Answers
Answer:
As a substance melts, and goes from a solid to a liquid state, the kinetic energy of the molecules increases, and the molecules move faster, and they separate further and further away from each other. The intermolecluar forces holding the molecules together become weaker. This is why a liquid can take fill the shape of its container, whereas a solid has a fixed shape.
Explanation:
take your notes man
Sarah wants to know where in her garden chamomile would grow the best. She thinks chamomile will grow best in the corner of the garden that gets the most sunlight. To test her hypothesis, she decides to plant several groups of chamomile in her garden as an experiment.
Answers
Answer:
This question is incomplete and lacks options, the complete part and the options are:
Which of the following variables should Sarah change from one group of chamomile to the next?
A. the location of the plants
B. the height of the plants
C. the type of plants
D. the amount of water she gives the plants
The answer is A
Explanation:
This question is asking for the INDEPENDENT VARIABLE in the experiment. The independent variable of an experiment is the variable that is changed or manipulated by the experimenter in order to bring about a response.
In this experiment where Sarah wants to know where in her garden chamomile plants would grow the best. She hypothesizes that chamomile will grow best in the corner of the garden that gets the most sunlight. However, to test this hypothesis, she decides to plant several groups of chamomile in her garden as an experiment. The variable that Sarah can change in the several groups of chamomile (independent variable) is the LOCATION OF THE PLANTS.
Note that, "where" is a question of location.
Answer:
The answer is C
Explanation:
what is the percent composition of salicylic acid?
Answers
The percent composition of salicylic acid is C7H6O3, or 60.87%C, 4.4%H, and 34.75%O
which two of these are also pure substances? Silicon
Brass
Gold
Steel
Rubber
Answers
are electric car batteries bad for the environment?
Answers
The environmental impact of electric car batteries depends on the source of the electricity used to charge them and the method of disposal or recycling of the batteries.
If the electricity used to charge the batteries comes from renewable sources, the environmental impact is minimal. However, if the electricity comes from fossil fuels, the environmental impact can be significant. Additionally, proper disposal or recycling of batteries is important to minimize environmental impact. The environment refers to the natural surroundings and conditions in which people, animals, and plants live. It includes everything from the air we breathe, the water we drink, the land we live on, and the weather patterns that affect us.
Learn more about environmental impact here:
https://brainly.com/question/29638468
#SPJ4
What is the atomic mass of potassium,sodium and neon?
Answers
Answer:
Pottasium : 39.0983uSodium : 22.989769uNeon : 20.1797u


Answer:
Potassium : 39.0983u
Sodium : 22.989769u
Neon : 20.1797u
To calculate the atomic mass of a single atom of an element, add up the mass of protons and neutrons
Hope this helps!
If 1000. mL of water freezes, which of the following is a reasonable approximation for the volume of the resulting ice?
Group of answer choices
1000. mL
961 mL
1040 mL
No answer text provided.
Previous Next
Answers
Answer:
If 1000. mL of water freezes, which of the following is a reasonable approximation for the volume of the resulting ice?
Group of answer choices
1000. mL
961 mL
1040 mL
Explanation:
Ice is fewer denser than water.
The reason is the volume occupied by the same mass of ice with water is more than the volume occupied by water. Ice has more empty space within it.
Due to this reason, ice floats on water.
When 1000ml of water freezes to ice then its volume is greater than water.
Among the given options the correct answer is 1040 mL .
Does NaCI have a high melting point
Answers
Answer:
Yes, at 801 °C
Explanation:
. describe how the ph of a solution relates to the hydrogen ion concentration. does a solution at ph 1 have more or less hydrogen ions than a solution at ph 4?
Answers
A solution at pH 1 has more hydrogen ions than a solution at pH 4. The pH of a solution refers to the hydrogen ion concentration.
The concentration of hydrogen ions and the pH of a solution are inversely proportional. This means that the higher the hydrogen ion concentration, the lower the pH, and vice versa.
A solution at pH 1 will have more hydrogen ions than a solution at pH 4.The pH of a solution is defined as the negative logarithm of the hydrogen ion concentration. The equation for calculating the pH of a solution is given as follows:
\($$pH = -\log_{10}[H^+]$$\)
In this equation, [H⁺] is the hydrogen ion concentration in moles per liter (mol/L) of solution. A change of 1 pH unit corresponds to a 10-fold change in the hydrogen ion concentration.
Therefore, if a solution has a pH of 1, it has a hydrogen ion concentration of 0.1 mol/L.
If a solution has a pH of 4, it has a hydrogen ion concentration of 0.0001 mol/L. Thus, a solution at pH 1 has more hydrogen ions than a solution at pH 4.
To know more about pH, refer
https://brainly.com/question/15265711
#SPJ11
Why is the wastewater that gets extracted alongside fracked hydrocarbons so salty?
Answers
The wastewater that is extracted alongside fracked hydrocarbons, also known as "produced water," is typically salty due to the high levels of dissolved minerals and salts present in the rock formations from which it is extracted.
When water is injected into the subsurface during hydraulic fracturing, it interacts with the rock formations and dissolves minerals such as sodium, calcium, and magnesium.
Additionally, the water used in hydraulic fracturing may contain additives such as salts and acids that can also contribute to the high salt content of produced water.
The high salt content of produced water poses significant environmental and economic challenges for its disposal and reuse. Treating and disposing of produced water can be costly, and improper disposal can lead to contamination of surface and groundwater resources.
Learn more about wastewater at
https://brainly.com/question/14219327
#SPJ4