Answers
Answer:
ye do it is bad for healthcare community has u to do with health to and public
Related Questions
name the chemical compound

Answers
Answer:
hydrochloric acid
Explanation:
chemical compound any substance composed of identical molecules consisting of atoms
How many moles of CO2 will be produced in the following reaction if the initial amount of reactants was 0.50 moles?
2NaHCO3 —> Na2CO3 + CO2 + H2O
Answers
Answer:
In the following reaction, how many moles of
CO2
can be made by reacting 0.50 moles of
CH4
with excess oxygen?
CH4+O2>CO2+H2O
Explanation:

A mixture of sodium hydroxide and strontium hydroxide contains a total of 0.19 moles of the two compounds. It requires 100.0 mL of 2.969 M HCl to neutralize all of the base. How many moles of sodium hydroxide were in the original mixture
Answers
Answer:
0.0831 moles of NaOH were in the original mixture
Explanation:
The strontium hydroxide, Sr(OH)2, requires 2 moles of HCl per mole of hydroxide to be neutralized.
The sodium hydroxide, NaOH, requires 1 mol of HCl per mol of hydroxide.
To solve this question we must write 2 equations:
(1) 0.19mol = X + Y
Where X = Moles NaOH; Y = Moles Sr(OH)2
The moles of HCl required are:
0.100L * (2.969mol / L) = 0.2969 moles HCl
0.2969 mol = X + 2Y(2)
Replacing (1) in (2):
0.19mol - Y = X
0.2969 mol = (0.19mol - Y) + 2Y
0.1069mol = Y
Moles X = Moles NaOH:
0.19mol = X + 0.1069mol
X = 0.0831 moles of NaOH were in the original mixture
Which statements describe inorganic compounds? Check all that apply
Inorganic compounds contain carbon
Inorganic compounds usually lack carbon
Inorganic compounds are not associated with or made from living things,
Inorganic compounds include fruits and vegetables,
Inorganic compounds include salt and water
Answers
Answer:
b, c, e
Explanation:
Inorganic compounds usually lack carbon.
Inorganic compounds are not associated with or made from living things.
Inorganic compounds include salt and water.
Answer:
yes the answer id bce
Explanation:
Cyclopropane is more reactive than most cycloalkanes. What factors lead to cyclopropane being less stable than the other cycloalkanes
Answers
Answer: The factor that lead to cyclopropane being less stable than the other cycloalkanes is the presence of a RING STRAIN.
Explanation:
In organic chemistry, the end carbon atoms of an open aliphatic chain can join together to form a closed system or ring to form cycloalkanes. Such compounds are known as cyclic compounds. Examples include cyclopropane, cyclobutane, cyclopentane and many among others.
Cyclopropane is less stable than other cycloalkanes mentioned above because of the presence of ring strain in its structural arrangement. The ring strain is the spatial orientation of atoms of the cycloalkane compounds which tend to give off a very high and non favourable energy. The release of heat energy which is stored in the bonds and molecules cause the ring to be UNSTABLE and REACTIVE.
The presence of the ring strain affects mainly the structures and the conformational function of the smaller cycloalkanes. cyclopropane, which is the smallest cycloalkane than the rest mentioned above, contains only 3 carbons with a small ring.
what are two elements that could displace iron in a single replacement reaction? how did you make this determination?
Answers
Two elements that could displace iron in a single replacement reaction are magnesium and zinc. This determination is made based on the reactivity series, which ranks metals according to their ability to displace other metals in aqueous solutions.
What is a single replacement reaction?An element replaces another element in a compound in a single replacement reaction, creating a new compound and a different element.
How does the reactivity series determine the displacement of metals in single replacement reactions?The reactivity series determines the displacement of metals in single replacement reactions by ranking metals in order of their reactivity, with the most reactive metal displacing less reactive metals from their compounds.
Magnesium and zinc, which are more reactive than iron, can displace iron in a single replacement reaction.
To know more about metals;visit:
https://brainly.com/question/28650063
#SPJ1
How many atoms of oxygen are on the reactants side of this balanced equation?
2AgNO3 (aq)+ Cu(s) —Cu(NO3)2 (aq)+ 2Ag(s)
12
2
3
6
Answers
Answer:
Explanation:
I tried to figure it out but my head wasn’t working sorry
A gas has a pressure of 0.370 atm at 323 K. What is the pressure at standard
temperature in K?
Answers
Answer:
0.0011455108359133
Explanation
323K=0.370atm
1K=0.370/323
Why do scientists' study and synthesize new transuranium elements in the laboratory?
Answers
Explanation:
Nuclear structure and stability
Although the decay properties of the transuranium elements are important with regard to the potential application of the elements, these elements have been studied largely to develop a fundamental understanding of nuclear reactions and nuclear and atomic structure.
Please help me do this

Answers
The total mass of the balloon and its content is 1521.17 g, the number of moles of CO₂ in the balloon is 34.15 mol, and the number of CO₂ molecules in the balloon is 2.06 x 10²⁵ molecules.
a) The molar mass of CO₂ is 44.01 g/mol. To find the total mass of the balloon and its content, we need to add the mass of the balloon (20g) to the mass of the CO₂ inside the balloon.
Mass of CO₂ = number of moles of CO₂ x molar mass of CO₂
Since the balloon is at STP (standard temperature and pressure), we can use the molar volume of a gas at STP (22.4 L/mol) to find the number of moles of CO₂ in the balloon:
Volume of CO₂ = Volume of balloon = 765 L (at STP)
Number of moles of CO₂ = volume of CO₂ / molar volume of a gas at STP
= 765 L / 22.4 L/mol
= 34.15 mol
Mass of CO₂ = 34.15 mol x 44.01 g/mol
= 1501.17 g
Total mass of balloon and its content = 20 g + 1501.17 g
= 1521.17 g
b) Number of moles of CO₂ in the balloon is 34.15 mol
c) To find the number of CO₂ molecules in the balloon, we need to use Avogadro's number (6.02 x 10²³ molecules/mol).
Number of CO₂ molecules = number of moles of CO₂ x Avogadro's number
= 34.15 mol x 6.02 x 10²³ molecules/mol
= 2.06 x 10²⁵ molecules
To know more about the Mass, here
https://brainly.com/question/22104139
#SPJ1
Your little sister asks you a scientific question: "Does chocolate milk come from brown cows?" In order to answer the question, you decide to form a hypothesis.
Explain whether or not the following statements are effective hypotheses.
i. Brown cows produce chocolate milk.
ii. Brown cows never produce chocolate milk.
iii. Brown cows produce white milk.
Answers
A hypothesis is a proposed explanation or prediction based on limited evidence or observations, which can be tested through further investigation or experimentation. It should be specific, testable, and based on existing knowledge.
Now, let's evaluate each statement as a hypothesis:Brown cows produce chocolate milk.This statement can be considered an effective hypothesis as it proposes a relationship between the color of cows and the color of milk they produce. It is specific and testable, as one could observe and analyze the milk produced by brown cows to see if it is indeed chocolate milk. However, based on existing knowledge, we can confidently say that this hypothesis is not accurate, as the color of a cow does not determine the color of the milk it produces.Brown cows never produce chocolate milk.This statement can also be considered an effective hypothesis because it makes a specific claim that can be tested. However, based on existing knowledge, we can say that this hypothesis is not accurate. While the color of a cow does not determine the color of the milk, it is possible for chocolate milk to be produced by adding chocolate syrup or cocoa powder to regular white milk.Brown cows produce white milk.This statement is not an effective hypothesis as it is a general statement that aligns with existing knowledge. It does not propose any specific relationship or prediction to be tested. In the context of this question, the statement is not accurate as milk produced by cows is typically white, regardless of their coat color.For such more question on hypothesis
https://brainly.com/question/606806
#SPJ8
the pressure on 20 milliliters of a gas at constant temperature is changed from 4 atmospheres to 2 atmospheres. what is the new volume of the gas?
Answers
The new volume of the gas whose pressure was changed would be = 40 milliliters.
How to calculate the new volume of the given gas?The initial volume(V1)of the gas= 20ml
The initial pressure(P1) = 4 atm
The final pressure(P2) = 2 atm
The final volume(V2) = ?
Using the general gas formula;
P1V1 = P2V2
V2 = P1V1/P2
= 4×20/2
= 40ml
Learn more about volume here:
https://brainly.com/question/27710307
#SPJ1
Calculate the pH of aqueous solutions having the following ion concentrations at 298 K [H+1 = 0.0055M
Answers
The pH of the given aqueous solution with [H+] = 0.0055 M at 298 K is approximately 2.26.
The formula used to find the pH of the solution is given as,
pH = -log[H+]
Substituting [H+] = 0.0055 M into the above formula, we get,
pH = -log(0.0055) ≈ 2.26
Therefore, the pH of the given aqueous solution with [H+] = 0.0055 M at 298 K is around 2.26.
A solution's acidity or basicity is determined by its pH. It is described as the hydrogen ion concentration's negative logarithm, pH = -log(H+). It is commonly expressed as [H+] and measured in units of moles per litre (M).
To know more about pH value, visit,
https://brainly.com/question/26424076
#SPJ1
Can one of you guys solve this whole page for me
Answers
Evolution can be defined as the change in genetic of any population over time.
Write something about Evolution?1. An adaptation is any heritable trait that helps an organism, such as a plant or animal, survive and reproduce in its environment.
2. Evolution is a process that results in changes in the genetic material of a population over time.
3. Natural selection, organisms that possess heritable traits that enable them to better adapt to their environment compared with other members of their species will be more likely to survive, reproduce, and pass more of their genes on to the next generation.
4. Charles Darwin is known as the father of evolution.
5. Darwin postulated that the beak of an ancestral species had adapted over time to equip the finches to acquire different food sources.
6. When a trait is expressed in more than one way, such as the different colours of the beetles, it is called variation.
7. A queen honeybee can lay up to 2000 eggs in one day. this is an example of overproduction of offspring.
8. In human beings, eye colour, eyebrows, earlobe size, and the ability to curl one's tongue are nonadaptive traits.
9. A dragonfly wing is the homologous structures.
10. Years of selective breeding by humans has resulted in the artificial "evolution" of dogs into many different types.
To learn more about Evolution, visit:
https://brainly.com/question/21202780
#SPJ1
The table describes a gas stored in four different containers. Properties of Stored Gas Container Properties 1 · Low number of collisions with container walls · Medium average kinetic energy · Large number of particles 2 · Large number of collisions with container walls · Medium average kinetic energy · Small number of particles with little spaces between them 3 · Large number of collisions with container walls · High average kinetic energy · Large number of particles with large spaces between them 4 · Few collisions with container walls · Low average kinetic energy · Small number of particles Which container has gas stored at the highest temperature? 1 2 3 4
Answers
Container 3 has the gas stored at the highest temperature.
Temperature is a measure of the average kinetic energy of the particles in a substance. In the given table, it is stated that container 3 has a large number of collisions with container walls, high average kinetic energy, and large number of particles with large spaces between them.
These properties indicate that the gas in container 3 has higher kinetic energy and more vigorous movement compared to the other containers.
Container 1 has a low number of collisions with container walls and a medium average kinetic energy. This suggests that the gas in container 1 has lower energy and less movement than the gas in container 3.
Container 2 has a large number of collisions with container walls, but it also has a small number of particles with little spaces between them. While the collisions may be frequent, the limited number of particles and the lack of space between them may result in lower overall kinetic energy compared to container 3.
Container 4 has few collisions with container walls, low average kinetic energy, and a small number of particles. These properties indicate that the gas in container 4 has the lowest energy and least movement among all the containers.
Container 3
For more such questions on temperature visit;
https://brainly.com/question/4735135
#SPJ8
Question 5 of 10
Which statement describes the law of conservation of energy?
A. When an energy transformation happens, no energy is destroyed
or created.
B. There is only one form of energy.
C. Energy can only change from nuclear energy to chemical energy.
D. The total energy in a system can only increase.
Answers
Answer:
A
Explanation:
A option is correct .
you cannot create energy neither you can destroy it but you can only convert into other form
The diagrams to the right show the distribution and arrangement of gas particles in two different containers. According to kinetic-molecular theory, which of the following statements is true? Check all that apply. If the temperatures of both containers are equal, container A has greater pressure than container B. If the volume of container A decreased, its pressure would decrease. If the pressure in both containers is equal, container A has a lower temperature than container B. Two containers are shown. Container A is square, and Container B is the same height, but is about twice as wide. Each container holds 6 gas particles distributed randomly.
Answers
The kinetic-molecular theory's true assertions are as follows:
If both containers' temperatures are the same, container A will have a higher pressure than container B.
Container A has a lower temperature than container B if the pressure in both containers is equal.
What is the kinetic theory of molecules?The molecules that make up a gas are always moving randomly, colliding with one another and the container walls, according to the kinetic molecular hypothesis. Remember that high temperature and low pressure are the only conditions in which perfect gases can exist.
The kinetic-molecular theory's true assertions are as follows:
If both containers' temperatures are the same, container A will have a higher pressure than container B.
If both are under pressure
Learn more about kinetic molecular theory:brainly.com/question/12025712
#SPJ1
Write a balanced chemical equation for the standard formation reaction of liquid chloroform
Answers
A balanced chemical equation for the standard formation reaction of liquid chloroform is :
CH₄ + 3Cl₂ -----> CHCl₃ + 3HCl
The methane combine with chlorine form the trichloro methane or the chloroform.
CH₄ + Cl₂ -----> CHCl₃ + HCl
to balance the equation , the number of atoms in the product side is equal to the reactant side :
reactant product
C 1 1
H 4 2
Cl 2 4
to balance the equation miltiply 3 in Cl₂ and 3 in HCl, we get :
CH₄ + 3Cl₂ -----> CHCl₃ + 3HCl
now the number of atoms in the product side is equal to the reactant side .
Thus, A balanced chemical equation for the standard formation reaction of liquid chloroform is :
CH₄ + 3Cl₂ -----> CHCl₃ + 3HCl
To learn more about balanced equation here
https://brainly.com/question/7181548
#SPJ1
PLEASE HELP, IS MY ANSWER CORRECT?
How does the ground temperature in sunlight with CO2 compare with the ground temperature in sunlight without CO2 (part A)? is my answer correct?
Based on the thermometer provided, it is clearly visible that when the simulation is without CO2, the temperature goes higher, however, not as quickly as when CO2 IS present.
Answers
Based on the thermometer provided, the ground temperature in sunlight with CO2 rises more rapidly and reaches a higher temperature compared to the ground temperature in sunlight without CO2.
PLS THIS IS DUE TODAY HELP ME LOOK AT THE PIC. like i beg you pls.

Answers
Answer:
kinetic energy is that form of mechanical energy that has been released and is associated with the body in motion.
Explanation:
My best answer
Can you solve this

Answers
Select the correct answer.
Iron oxide reacts with aluminum to give aluminum oxide and iron. What kind of chemical reaction is this?
Answers
Answer:
Single-replacement reaction
Explanation:
Single-replacement reactions, aka. single-displacement reactions, involve one element/ion in a compound being replaced by another element/ion. In this case, aluminum is replacing the iron in the compound.
If you need help visualizing, the equation looks like this:
Fe₂O₃ + Al³⁺ --> Al₂O₃ + Fe³⁺
How many grams of phosphorus are in a sample containing 10^30 phosphorus atoms?
Answers
Answer:
18.1 g
Explanation:
You know that the atomic weight of phosphorus is equal to
30.794 u
, where
u
represent the unified atomic mass unit.
The unified atomic mass unit is equivalent to
1 g/mol
, but let's take the long road and prove that identity.
Now, the unified atomic mass unit is defined as
1
12
th
of the mass of a single unbound carbon-12 atom in its ground state and is equivalent to
1 u
=
1.660539
⋅
10
−
24
g
This means that the mass of one phosphorus atom will be
30.974
u
⋅
1.660539
⋅
10
−
24
g
1
u
=
5.14335
⋅
10
−
23
g
You know that one mole of any element contains exactly
6.022
⋅
10
23
atoms of that element - this is known as Avogadro's number.
Well, if you know the mass of one phosphorus atom, you can use Avogadro's nubmer to determine what the mass of one mole of phosphorus atoms
5.14335
⋅
10
−
23
g
atom
⋅
6.022
⋅
10
23
atoms
1 mole
=
30.974 g/mol
Finally, if one mole of phosphorus atoms has a mass of
30.974 g
, then
0.585
moles will have a mass of
0.585
moles
⋅
30.974 g
1
mole
=
18.1 g
From: https://socratic.org/questions/the-atomic-weight-of-phosphorus-is-30-974-u-what-is-the-mass-of-a-phosphorus-sam
explain how the solid liquid line in the phase diagram of water differs in character from the solid liquid line in the phase diagrams of most other substances such as CO2
Answers
Answer:
The solid-liquid line in the water phase diagram has a negative slope, whereas for most other substances it has a positive slope.
The ice has a lower density as compared to water still they have in the solid phase.
Reason how the solid-liquid line in the phase diagram as compared to other:In the solid-liquid line in the water phase diagram it contains the negative slope. While the most other substance should have the positive slope.
Also, when the pressure is applied so the higher density should be favored. In the water, the highest density phase should be likely the liquid water.
Learn more about liquid here: https://brainly.com/question/17109464
Which example is a biotic factor of an aquarium ecosystem?
A) gravel on the bottom of the aquarium
B) algae growing on the glass
C) plastic plants placed in the gravel
D) rock structure
Answers
An example of a biotic factor of an aquarium ecosystem is an algae growing on the glass. Option B is the correct answer.
What is a biotic factor?This refers to a living organism that shapes its environment. The examples of biotic factor that make up fresh water ecosystem are: aquatic plants, fish, amphibians,fungi, bacteria, and protists. and algae.
The biotic factors are gropped into : producers, consumers, and decomposers.
The producers, convert abiotic factors into food. They include plants and algae.The consumers do not make their own food .They consume producers or other consumers to get food energy.The decomposers are the organisms that break down organic matter from dead plants and animals into the inorganic components, for example carbon and nitrogen, which are important for life.Learn more about biotic factor on
https://brainly.com/question/29776080
#SPJ1
the carbon skeletons of which two amino acids enter metabolism at the level of pyruvate? choose the best answer(s) from the list below. 1- alanine and serine 2- alanine and cysteine 3- serine and asparagine 4- alanine and aspartate
Answers
The best answer 1 and 2, the carbon skeletons of which two amino acids enter metabolism at the level of pyruvate are alanine and serine or alanine and cysteine.
Metabolism refers to the chemical processes that occur within an organism to maintain life. These processes include the breakdown of nutrients to release energy and the synthesis of molecules that the body needs for growth and repair. There are two main types of metabolism: catabolism, which involves the breakdown of molecules to release energy, and anabolism, which involves the synthesis of molecules using energy.
The rate at which an individual's metabolism operates can affect their weight, with a faster metabolism generally leading to a higher rate of calorie burn and a slower metabolism leading to a lower rate of calorie burn. However, factors such as genetics, age, and sex also play a role in determining an individual's metabolism. Additionally, lifestyle factors such as diet and physical activity can also impact metabolism, with regular exercise and a balanced diet known to help boost metabolism.
To learn more about Metabolism visit here:
brainly.com/question/630824
#SPJ4
What electrons participate in chemical bonding?
Answers
Answer:
valence electrons
Explanation:
they are the electrons that are in the outermost shell
What's the molality of a solution with 120 g of NaCl and 30 kg of water?A)0.068B)0.004C)0.25D)6.8
Answers
Explanation:
Molality is the ratio of the amount of substance of a solute (in moles – n) to the mass in kilograms of the solvent (m).
The symbol for molality is usually W and it can be calculated by the following formula:
W = n/m
here, we need to calculate n of NaCl using the formula:
n = m/MM
where MM of NaCl is 58.5 g/mol
n = 120/58.5
n = 2.05 moles
W = 2.05/30
W = 0.068 mol/Kg
Answer: A) 0.068
Prepare one solution that has 0.12 M of FeCl3 and 0.40 M of HCl with the reagents 3 M HCl and Solid FeCL3 * 6H20. Provide the calculations and protocol to make the solution in a lab.
Answers
To prepare a 0.12 M solution of FeCl₃, the amount of solid FeCl₃ to be dissolved in a given volume of solvent will be 9.72 grams.
Given,
Molarity of FeCl₃ (M)= 0.12 M
The molecular weight (m) of FeCl₃ is = 162 gm
The volume of the solution (V) to be prepared is =500 ml
The amount of FeCl₃ to be dissolved to make a 0.12 M solution is= x
So,
MV= x ÷ m × 1000
0.12× 500 = x ÷ 162 × 1000
x = 60 × 162 ÷ 1000
x= 9.72 gm
So 9.72 grams of FeCl₃ is dissolved to make 500 ml of 0.12 M solution.
For preparing 0.4 M HCl from 4M HCL:
If we need to make 500 ml of solution with 0.4M of HCL, then we use the formula:
M₁V₁= M₂V₂
0.4 × 500= 4 × x
x= 50 ml
So 50 ml of 4M HCL is taken to make 0.4 M HCL.
To learn more about FeCl₃, refer to the link:
https://brainly.com/question/32098087
#SPJ1
Please answer .
Which answer choice is correct ?
There are four options.
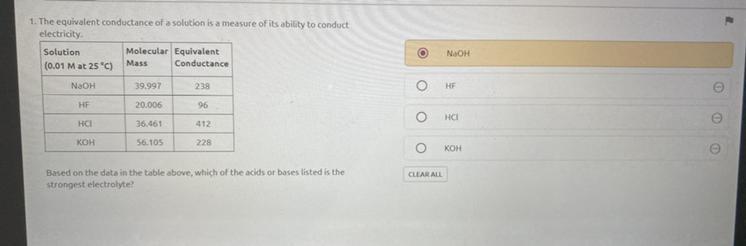
Answers
Answer:
I also think NaOH is the answer since they are all strong electrolytes and NaOH has the highest number