Answers
Answer:
Al°(s) + 3Ag⁺(aq) => Al⁺³(aq) + 3Ag(s)
Explanation:
Oxidation: Al°(s) => Al⁺³(aq) + 3e⁻
Reduction: 3Ag⁺(aq) + 3e⁻ => 3Ag°(s)
_________________________________________
Net Rxn: Al°(s) + 3Ag⁺(aq) => Al⁺³(aq) + 3Ag(s)
One mole of neutral aluminum atoms (Al°(s)) undergo oxidation delivering 3 moles of electrons to 3 moles silver ions (3Ag⁺³(aq)) that are reduced to 3 moles of neutral silver atoms (3Ag°(s)) in basic standard state 25°C; 1atm.
A balanced equation obeys the law of conservation of mass. According to the law of conservation of mass, mass can neither be created nor be destroyed. The coefficient of silver is 3.
What is a balanced equation?A balanced chemical equation can be defined as the chemical equation in which the number of reactants and products on both sides of the equation are equal. The amount of reactants and products on both sides of the equation will be equal in a balanced chemical equation.
The numbers which are used to balance the chemical equation are called the coefficients. The coefficients are the numbers which are added in front of the formula.
The balanced chemical equation for the given redox reaction is given as:
Al (s) + 3 Ag⁺ (aq) → Al³⁺ (aq) + 3Ag (s)
Thus the coefficient of silver is 3.
To know more about balanced equation, visit;
https://brainly.com/question/29769009
#SPJ7
Related Questions
Which of the following is not one of the four things that all organisms need to survive?
A. water
B. air
C. a source of energy
D. the ability to move
Answers
Answer:
b
Explanation:
this is because all organisims need water, c is out of the question because no energy is needed, d is out because it is needed for fighting against predators
does a sailboat or a submarine has more momentum
Answers
Answer: Submarine
Explanation:
Depends on the size and weight of the sailboat and submarine but the submarine will have more momentum because it is heavier.
Explain with the help of an example the reaction exhibited by carbonyl compounds having at least one α-hydrogen.
Answers
Aldehydes and ketones are organic compounds which incorporate a carbonyl functional group, C=O. The carbon atom of this group has two remaining bonds that may be occupied by hydrogen or alkyl or aryl substituents. If at least one of these substituents is hydrogen, the compound is an aldehyde. If neither is hydrogen, the compound is a ketone.
Carbonyl compounds having at least one α-hydrogen undergo nucleophilic addition as shown below:
CH₃HC=O + H₂0 + H+ ----> CH₃CH(OH)₂What are carbonyl compounds?Carbonyl compounds have a carbon atom bonded to oxygen by means of a double bond.
The two other bonding positions are occupied by two R groups or a hydrogen atom and an R group as in aldehydes.
Carbonyl compounds having at least one α-hydrogen undergo nucleophilic addition.
For example, nucleophilic addition of water to ethanal:
CH₃HC=O + H₂0 + H+ ----> CH₃CH(OH)₂
Learn more about carbonyl compounds at: https://brainly.com/question/10135014
Which type of energy transformation occurs when a flashlight shines?

Answers
Answer:
chemical,electrical,light
Explanation:
The battery has chemical energy. When the flashlight is turned on, the chemical energy is first transformed into electrical energy and then into light energy.
Good Luck :)
Chemical, electrical, light
Chemical energy is the energy released by chemical substances as they undergo a chemical reaction.Electrical energy is the energy produced by the movement of electrically charged particles.Light energy is a type of electromagnetic radiation that has wavelengths.Chemical energy is stored in the battery. When you turn on the flashlight, chemical energy is converted into electrical energy, which is subsequently converted into light energy.Type of energy transformation that occurs when a flashlight shines is "Chemical, electrical, light"For more information:
https://brainly.com/question/19040689?referrer=searchResults
The Colstrip Power Plant
in Montana burns _______
releasing _______
into the air.
A. Iron; HCI
B.Carbon; base(soap)
C. Coal; H2SO4(sulfuric acid)
D. None of the above
Answers
Answer:
Coal; H2SO4(sulfuric acid)
Explanation:
Coal has been very helpful in power generation for decades. It has also become a major contributor to global warming, and has major negative effects on human health and the environment.
Coal is formed when dead plant matter submerged in swamp environments is subjected to the geological forces of heat and pressure over hundreds of millions of years. Over time, the plant matter transforms from moist, low-carbon peat, to coal, an energy- and carbon-dense black or brownish-black sedimentary rock.
There are many types of coal. Each type of coal must contain sulfur, which, when burned, releases toxic air pollution. The extent Sulfur content in a given coal sample is largely decided by the conditions under which the coal is formed. Low-sulfur coal usually develop in freshwater environments; high-sulfur coals are formed in brackish swamps.
The burning of coal releases its sulphur content as oxides of sulphur (SOx). These oxides of sulphur dissolve in rain water to form acid rain. Hence rain falling around the Colstrip power plant will contain H2SO4 resulting from the dissolution of SOx in rain droplets.
Why was meteorology such a late developer compared to other branches of science?
Answers
Answer:
Because of the difficulties of measuring the atmosphere's properties above the earth's reachable surface
Explanation:
Hello,
In this case, meteorology is the branch of science studying the atmosphere in its weather processes and forecasting and it had a late development because of the difficulties of measuring the atmosphere's properties above the earth's reachable surface. We cannot forget that even nowadays, it is very difficult to predict upcoming weathers with the 100 % assurance and with many days in advance.
Best regards.
Meteorology is developed lately as compared to other branches of science due to far away from the reach of humans.
Meteorology is a late developer compared to other branches of science because the measuring the climatic conditions in the atmosphere is difficult and even impossible without the presence of advance technologies.
To find out the weather as well as climatic conditions can't be measured due to it is not reachable to the human like other materials present on the earth surface so we can conclude that meteorology is developed lately as compared to other branches of science due to far away from the reach of humans.
Learn more: https://brainly.com/question/16565664
Why does the light coming from a flashlight appear brighter when it is closer to an object than when it is farther
away?
a. It is because the air is absorbing less of the light.
b. It is because the air is absorbing more of the light.
c.It is because the light waves are being refracted.
d. It is because the light waves are appearing at their normal line.
Answers
The answer is c and possibly a I don't know why im here i dont know what this is so bye...
Explanation:
C I hope this is right for your question.
What is the balanced form of the chemical equation shown below?
C₂H5OH() + O₂(g) → H₂O(A) + CO₂(g)
Answers
Answer: C(2)H(5)OH + 3O(2) —> 3H(2)O + 2CO(2)
Explanation:
*The number in parentheses or
( ) represents the subscript of the element
Carbon-2
Hydrogen-6
Oxygen-7
I hope this is helpful.
Blood is buffered by carbonic acid and the bicarbonate ion. Normal blood plasma is 0.024 M in HCO3- and 0.0012 M H2CO3 (pka for H2CO3 at body temperature is 6.1).
a) What is the pH of blood plasma?
b) If the volume of blood in a normal adult is 5.0L, what mass of HCl could be neutralized by the buffering system in blood before the pH falls below 7.0 (which would result in death)?
c) Given the volume from part (b), what mass of NaOH could be neutralized before the pH rises above 7.8?
Answers
Before the pH drops below 7.0, the buffering mechanism in blood can neutralise 1.26 kg of HCl.
(a) The equilibrium expression for the reaction between H₂CO₃ and HCO₃₋ is:
H₂CO₃ ↔ H+ + HCO₃₋
The pKa for H2CO3 at body temperature is 6.1, so the pH of blood plasma can be calculated using the Henderson-Hasselbalch equation:
pH = pKa + log([HCO₃₋]/[H₂CO₃])
pH = 6.1 + log(0.024/0.0012)
pH = 7.4
Therefore, the pH of blood plasma is 7.4.
(b) The buffering capacity of blood can be calculated using the equation:
buffering capacity = Δ[HCO₃₋]/ΔpH
ΔpH is the change in pH that can be tolerated before the buffering system is overwhelmed. The buffering capacity of blood is highest at a pH of 7.4, which is the normal pH of blood plasma. Therefore, we will use a pH range of 7.4 ± 0.6 (i.e., from 6.8 to 8.0) to calculate the buffering capacity.
At pH 6.8, the [H+] is 10 times higher than at pH 7.4, so [H+] = 10^-6.8 = 1.58 × 10^-7 M.
At pH 8.0, the [H+] is 10 times lower than at pH 7.4, so [H+] = 10^-8.0 = 1.00 × 10^-8 M.
The change in [HCO3-] can be calculated using the Henderson-Hasselbalch equation:
[HCO₃₋] = [H₂CO₃]/(1 + 10^(pH-pKa))
At pH 6.8:
[HCO₃₋] = 0.0012/(1 + 10^(6.8-6.1)) = 0.0006 M
At pH 8.0:
[HCO₃₋] = 0.0012/(1 + 10^(8.0-6.1)) = 0.011 M
Δ[HCO₃₋] = 0.011 - 0.0006 = 0.0104 M
Therefore, the buffering capacity of blood is:
buffering capacity = 0.0104/0.6 = 0.0173 M/pH
The amount of HCl that can be neutralized before the pH falls below 7.0 can be calculated using the buffering capacity:
[H+] = 10⁻⁷ = 1.00 × 10⁻⁷ M
Δ[HCO₃₋] = buffering capacity × ΔpH = 0.0173 × (7.4 - 7.0) = 0.0069 M
The amount of HCl that can be neutralized is:
mass of HCl = Δ[HCO₃₋] × volume of blood × molar mass of HCl
mass of HCl = 0.0069 × 5.0 × 10^3 × 36.5 = 1.26 × 10³ g
Therefore, the buffering system in blood can neutralize 1.26 kg of HCl before the pH falls below 7.0.
To learn more about ph refer to:
brainly.com/question/15289741
#SPJ4
Your weather app reports the actual temperature as 34°F but the temperature on the thermometer outside is 35°F. What is the percent error of the recorded temperature (absolute error = ±1°F).

Answers
The percentage error of the recorded temperature is 2.9%
Formula for percentage errorPercentage error = (absolute error / actual measurement) × 100
Data obtained from the questionThe following data were obtaned from the question:
Actual temperature reading = 34 °FThermometer reading = 35 °FAbsolute error = 1 °FPercentrage error =?How to determine the percentage errorThe percentage error of the recorded temperature can be obtained as follow:
Percentage error = (absolute error / actual measurement) × 100
Percentage error = (1 / 34) × 100
Percentage error = 2.9%
Thus, the perecentage error of the recorded temperature is 2.9%
Learn more about percentage error:
brainly.com/question/11290033
#SPJ1
Summarize the physical and chemical properties of zinc
Answers
Characteristics of the element:
density – 7.13 g/cm³;
color – bluish-white;
melting point – 420 °C;
elasticity and malleability increase when heated to approximately 100 °C;
boiling point of 906 °C;
at temperatures above 200 °C, loses its elasticity and becomes a grey powder;
high heat capacity and heat conductivity;
good conductor.
Chemical properties of the element
In ordinary conditions, zinc reacts rapidly with air, gradually forming a dull grey zinc oxide coating. Additionally, zinc reacts with halogens, oxygen, chalcogens, alkalis, acids, ammonia and ammonium salts, and even with less active metals. Zinc reacts with both acids and alkalis, making it an amphoteric metal. When reacting with alkalis, the element forms complex compounds known as hydroxo-zincates.
If you have a 350 gram sample of an isotope with a four-year half-life, how much of the sample would remain after three half-lives? Explain your reasoning.
Answers
After one half life initial amounts gets half so :-
First half life :- 350÷2 = 175 grams second half life :- 175 ÷2 = 87.5 grams third half life :- 87.5 ÷2 = 43.75 gramsso at last of third half life amount left = 43.75 grams
each time amount get half
ask ure doubt freely in comments
An atom has the electron configuration shown below. How many valence electrons are in this atom?
Captionless Image
2
3
4
6
What charge would you expect on an ion of this fictitious atom?
Captionless Image
+3, cation
+5, cation
-5, anion
-3, anion
Which occurrence explains why noble gases are essentially unreactive?
Dipole Interactions
VSEPR Theory
Dispersion Forces
Octet Rule
A covalent bond occurs when ________ are shared between two atoms.
electrons
protons
neutrons
dipoles.
A substance conducts electricity well and has a boiling point of 1478 °C. Is this likely an ionic or covalent substance?
Not enough information to answer.
ionic
covalent
Select all the compounds listed below that are covalent compounds.
B
C
D
A
Captionless Image
A
B
C
D
Captionless Image
Trihocus Dipocus
Dihocus Tripocus
Hocus (II) Pocus
Hocus (III) Pocus
Two nonmetal Halloween elements react to form the compound TriTrick DiTreat. What is the formula? Trick = X and Treat = Y
C
D
B
A
Write the formula for Candycorn Pumpkinate. Candycorn (Cc) has a charge of +3 and pumpkinate (PkO) a charge of -2.
A
B
C
D
Which statement best describes the atomic structure of metals?
Valence electrons packed in a lattice arrangement around cations.
Valence electrons packed in a lattice arrangement around anions.
Closely packed cations with loosely held valence electrons.
Loosely packed cations with tightly held valence electrons.
Which of the following properties is NOT explained by metallic bonding?
Crystalline structures
Malleabilty
Ductility
Thermal conductivity
What is the ionic charge of element X in the compound XP? Phosphorus is in group 15 on the periodic table.
1+
1-
3+
3-
_____________is not reactive with only 2 electrons in its valence shell.
Krypton
Helium
Neon
Xenon
How many electrons are shared in the double bonds in the structural formula shown below?
Captionless Image
2
4
6
8
Option 5
How many lone PAIRS are shown in the molecule below?
Captionless Image
none
2
4
8
A molecule of XY (2 nonmetals) is polar because
X is a metal so exhibits magnetic properties.
X and Y are both ions.
it is ionic and all ionic bonds are polar.
the X atom is able to attract the shared electrons more strongly than a Y atom.
Answers
Answer:
question no 4 answer is electrons
Perform the following calculations with the correct significant figures. Show your initial, calculator answer and your final answer. Example: 2.50 cm * 4.00 cm = 10 cm^2= 10.0 cm^ 2 or 1.00 * 10^1 cm^2 A) 3.182 cm * 0.503 cm = B) 0.1567 g + 2.10 g + 1.203 g = C) 8.109 mL – 2.13 mL = D) 9.843 g/2.35 mL =
Answers
Answer:
A) 1.60. cm².
B) 3.46 g.
C) 5.98 mL.
D) 4.19 g/mL.
Explanation:
Hello,
In this case, considering that the result is displayed by showing the same significant figures that the shortest number has and considering the eventual rounding when the next number is greater than 5, we obtain:
A) 3.182 cm * 0.503 = 1.600546 cm² but the number is shown with three significant figures (same as 0.503): 1.60 cm².
B) 0.1567 g + 2.10 g + 1.203 g = 3.4597 g but the number is shown with three significant figures (same as 2.10 g): 3.46 g.
C) 8.109 mL – 2.13 mL = 5.979 mL but the number is shown with three significant figures (same as 2.13 mL): 5.98 mL.
D) 9.843 g/2.35 mL = 4.188511 g/mL but the number is shown with three significant figures (same as 2.35 mL): 4.19 g/mL.
Regards.
The rate of effusion of NH3 is 2.40 mole/min. What would be the rate of effusion of He under the same conditions?
Answers
The rate of effusion of helium under same conditions as that of ammonia is 4.897 mole/min.
Rate of effusion of ammonia is 0.49 times the effusion of helium.Thus, to find out effusion of helium ,effusion of ammonia is divided by 0.49.
Thus, effusion of helium=effusion of ammonia/0.49
=2.40/0.49
=4.897 mole/min.
What is rate of effusion?Effusion relates to the ability of gas to travel through a small opening.Rate of effusion of a gaseous substance is inversely proportional to square root of it's molar mass.The relationship between molar mass and rate of effusion is called Graham's law.
Effusion rate directly depends on the average velocity with which a particle moves.Higher the velocity of particles,higher is the effusion rate.
Learn more about effusion ,here:
https://brainly.com/question/28320456
#SPJ1
How does heat affect water and its state of matter
Answers
Answer:If a liquid is heated the particles are given more energy and move faster and faster expanding the liquid. The most energetic particles at the surface escape from the surface of the liquid as a vapour as it gets warmer. Liquids evaporate faster as they heat up and more particles have enough energy to break away.
Explanation:
When you decide whether or not the data supports the original hypothesis, you are
O creating a theory
forming a hypothesis
O contributing to the body of knowledge
stating a law
O asking questions
Answers
Contributing to the body of knowledge.
When you decide whether or not the data supports the original hypothesis, you are "contributing to the body of knowledge." Explanation:Scientific investigation is a way of answering questions about the natural world. An inquiry or investigation can be initiated by a researcher or a group of researchers who have questions regarding a certain phenomenon. The inquiry or investigation is usually done by conducting an experiment or making an observation and then collecting data. After collecting the data, the researchers analyze it to check if it supports their hypothesis or not.The body of knowledge refers to the totality of data that has been collected and analyzed. It comprises all the data that researchers have acquired over time through scientific investigations. When researchers decide whether or not the data supports their original hypothesis, they contribute to this body of knowledge. The new data may either confirm the hypothesis or lead to a revision or rejection of it, adding to the body of knowledge.
for more questions on knowledge
https://brainly.com/question/29610548
#SPJ8
How many atoms are in the compound described by this chemical formula?
(CH3)2N
Answers
There are 8 atoms in the compound described by this chemical formula. There are 2 Carbon atoms (C), 6 Hydrogen atoms (H), and 1 Nitrogen atom (N).
What is atom?Atom is the smallest unit of matter that makes up all materials. It is composed of a nucleus, which contains protons and neutrons, surrounded by electrons. All elements consist of different combinations of atoms. Atoms can combine in various ways to form molecules, which are the basis of all matter on Earth. In chemistry, atoms are the building blocks of matter and are essential for understanding the structure and properties of different substances. Additionally, atoms can be used to understand the interactions between elements and molecules, the chemical reactions that occur between them, and the changes in energy that occur during these reactions.
To learn more about atom
https://brainly.com/question/28449914
#SPJ1
1
Select the correct answer from each drop-down menu.
Complete the following statements to describe solids, liquids, and gases. Select the correct answer from each drop-down menu.
A solid
A liquid
A gas
а a definite volume and
a definite volume and
a definite volume and
a definite shape.
a definite shape.
a definite shape.
Answers
From each drop-down menu, a solid has (a definite volume and a definite shape), a Liquid has (a definite volume) and gas has ( non of the above)
The features of different states of Matter:Matter is defined as anything that has weight and occupies space.
There are three states of matter that is in existence which include:
Solid: The particles of solid are closely packed together and vibrate around fixed axes. That is why they have a definite shape and volume.Liquid: The particles of liquid, though attracted to each other,move freely over each other. That is why they have definite volume but not a definite shape.Therefore, a liquid occupies the shape of its container.
Gas: The particles of gas contain scattered molecules that are dispersed across a given volume.Therefore, a gas neither has a definite shape nor volume.
Learn more about matter here:
https://brainly.com/question/3998772
When zinc reacts with copper sulfate solution, zinc sulfate solution and copper are formed.(i) An experiment was carried out to measure the temperature change when zinc powder reactswith copper sulfate solution.initial temperature of copper sulfate solution = 20 °Cfinal temperature of mixture after the reaction = 46 °CExplain what the temperature readings show about the type of heat change that occurs duringthis reaction.
Answers
The temperature increase from 20 °C to 46 °C indicates that the reaction between zinc and copper sulfate solution is exothermic, with heat being released into the surroundings.
In the given reaction between zinc and copper sulfate solution, the temperature change can provide insights into the type of heat change occurring during the reaction. Based on the provided information, the initial temperature of the copper sulfate solution was 20 °C, and the final temperature of the mixture after the reaction was 46 °C.
The temperature increase observed in this reaction indicates an exothermic heat change. An exothermic reaction releases heat energy into the surroundings, resulting in a temperature rise. In this case, the reaction between zinc and copper sulfate solution is exothermic because the final temperature is higher than the initial temperature.
During the reaction, zinc displaces copper from copper sulfate to form zinc sulfate and copper metal. This displacement reaction is known as a single displacement or redox reaction. Zinc is more reactive than copper and therefore replaces copper in the compound.
The formation of new chemical bonds during the reaction releases energy in the form of heat. This energy is transferred to the surroundings, leading to an increase in temperature. The heat released is greater than the heat absorbed, resulting in a net increase in temperature.
The exothermic nature of this reaction can be explained by the difference in bond energies between the reactants and products. The breaking of bonds in the reactants requires energy input, while the formation of new bonds in the products releases energy.
In this case, the energy released during the formation of zinc sulfate and copper metal is greater than the energy required to break the bonds in copper sulfate and zinc.
For more such question on temperature visit:
https://brainly.com/question/4735135
#SPJ8
Explain two positive aspects of using methane recapture systems.
Answers
Answer:
Two positive aspects of using methane recapture systems are able to generate significant electricity. Another benefit is that the process of anaerobic digestion creates heat that can be used to warm buildings where animals are kept
Answer: The correct answer is;
Two positive aspects of using methane recapture systems include lowering the impact on greenhouse gasses and the production of energy. Methane is a very potent greenhouse gas that is contributing to global warming. As a result, the recapturing process reduces the methane impacts of global warming by reclaiming and reusing the gas for other purposes. Recaptured methane can be stored and used to generate electricity or used as fuel to power updated vehicles and other engines on the farm. The overall benefits from this combination are reducing impacts causing global warming and lower the cost of electricity or fuel on the farm.
Explanation: This answer has been confirmed correct.
Balance the equation by inserting coefficients as needed. equation: C 2 H 6 O+O 2 longrightarrow CO 2 +H 2 O
Answers
The coefficients needed to balance the equation would be 2, 7, 4, and 6 respectively.
Balancing chemical equationThis requires that the number of atoms of each element is the same both on the reactant and product's sides.
Hence, the balanced equation of the reaction becomes:
\(2C_2 H_6 O+ 7O_2 --- > 4CO_2 +6H_2 O\)
Thus, the coefficient for each species would be 2, 7, 4, and 6 respectively.
More on balancing chemical equations can be found here: https://brainly.com/question/15052184
#SPJ1
Identify the missing coefficient in the balanced equation and classify the type of reaction. C2H5OH + 3O2 ⟶ _____CO2 + 3H2O 2; Combustion 2; Neutralization 3; Combustion 3; Neutralization
Answers
Answer:
It is a combustion reaction
Explanation:
I'm not sure about the number, but for sure it's a combustion reaction because the products are water and co2.
9) For the balanced equation (with hypothetical
2A + 3B
[B] (mol/L)
0.100
0.100
0.200
Exp#
1
2
3
[A](mol/L)
0.100
0.200
0.100
a. What is the order for each reactant?
b. What is the overall order for the reaction?
C + 4D
initial rate (M/sec)
0.022
0.176
0.044
Answers
The order for reactant A is 2 and the order for reactant B is 1. For the first reaction, the overall order of the reaction is 3 and for the second reaction, the overall order of the reaction is 5.
What is the order of a reaction?The order of a reaction is the sum of the exponents in the rate law expression that relates the rate of a chemical reaction to the concentrations of the reactants.
To determine the order of each reactant, we need to compare the initial rates of reaction at different concentrations while keeping the concentration of the other reactant constant.
For reactant A:
Exp#1 (0.100 M A, 0.100 M B): initial rate = k(0.100)^2(0.100) = 0.001 k
Exp#2 (0.200 M A, 0.100 M B): initial rate = k(0.200)^2(0.100) = 0.004 k
Exp#3 (0.100 M A, 0.200 M B): initial rate = k(0.100)^2(0.200) = 0.002 k
We can see that when the concentration of A doubles (Exp#1 to Exp#2), the initial rate quadruples, which indicates that A is second order. When the concentration of B doubles (Exp#1 to Exp#3), the initial rate doubles, which indicates that B is first order.
Therefore, the order for reactant A is 2 and the order for reactant B is 1.
To determine the overall order of the reaction, we add the orders of the reactants:
Overall order = 2 (order of A) + 1 (order of B) = 3
Therefore, the overall order of the reaction is 3.
For the second reaction, we can see that the rate depends on the concentration of both reactants, and we cannot determine their individual orders without further information or experiments. However, we can determine the overall order of the reaction by adding the exponents of the concentration terms in the rate law:
Overall order = 1 + 4 = 5
Therefore, the overall order of the reaction is 5.
Learn more about order here:
https://brainly.com/question/13467963
#SPJ1
Which bone is located between the incus and the inner ear?
cochlea
stapes
incus
malleus
Answers
Answer: The answer is incus
According to the data, which part is described by the letter K?
a
constellation
b
galaxy
c
meteoroid
d
planet
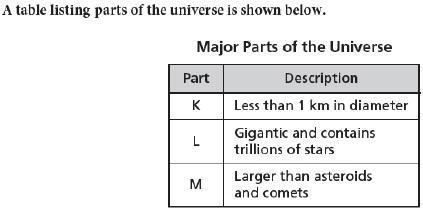
Answers
Answer:
C meteoroid
Explanation:
Meteoroids are the only ones of the four that are under a kilometer in size.
Determine the volume (in mL) of 1.00 M NaOH that must be added to 250 mL of 0.50 M CH3CO₂H to produce a buffer with a pH of 4.50.
Answers
Approximately 70.57 mL of 1.00 M NaOH should be added to 250 mL of 0.50 M CH3CO₂H to produce a buffer with a pH of 4.50.
To determine the volume of 1.00 M NaOH required to produce a buffer with a pH of 4.50 when added to 250 mL of 0.50 M CH3CO₂H, we need to consider the Henderson-Hasselbalch equation and the stoichiometry of the reaction.
The Henderson-Hasselbalch equation for a buffer solution is given as:
pH = pKa + log([A-]/[HA])
In this case, CH3CO₂H (acetic acid) acts as the weak acid (HA) and CH3COO- (acetate ion) acts as its conjugate base (A-). We are given that the desired pH is 4.50, and we can determine the pKa value for acetic acid from reference sources, which is approximately 4.75.
Using the Henderson-Hasselbalch equation, we can rearrange it to solve for the ratio [A-]/[HA]:
[A-]/[HA] = 10^(pH - pKa)
[A-]/[HA] = 10^(4.50 - 4.75) = 10^(-0.25) = 0.5623
This means that the ratio of the acetate ion to acetic acid in the buffer solution should be approximately 0.5623.
To calculate the required volume of NaOH, we need to consider the stoichiometry of the reaction. Acetic acid reacts with hydroxide ions (OH-) to form acetate ions and water:
CH3CO₂H + OH- → CH3COO- + H2O
The stoichiometric ratio between acetic acid and hydroxide ions is 1:1. Therefore, the volume of 1.00 M NaOH needed can be calculated using the equation:
Volume (NaOH) × 1.00 M = Volume (CH3CO₂H) × 0.50 M × 0.5623
Volume (NaOH) = (Volume (CH3CO₂H) × 0.50 M × 0.5623) / 1.00 M
Volume (NaOH) = (250 mL × 0.50 M × 0.5623) / 1.00 M
Volume (NaOH) ≈ 70.57 mL
For more such questions on buffer visit:
https://brainly.com/question/13076037
#SPJ8
What is the oxidation state of P in PO43-?
O A. +8
O B. +3
O C. +5
OD. +2
Answers
Answer:
the answer is +5
Explanation:
good luck :)
Balance the following chemical equations.
Zn + HCI -> H2+ZnCI2
CS2+O2 -> CO2 + SO2
30 POINTS FOR ALL
if its incomplete or wrong ill report you lol
Answers
Answer:
Zn + 2HCl -> H2 + ZnCl2
CS2 + 2O2 -> CO2 + S2O2
Explanation:
how many molecules of potassium chloride will react if 21.89 grams KCl are added to the solution
Answers
There are approximately 1.765 x 10²³ molecules of KCl in 21.89 grams of KCl.
What is meant by potassium chloride ?Potassium chloride (KCl) is a compound made up of potassium and chloride ions. It is a colorless, odorless salt that is commonly used in a variety of applications.
Molar mass of KCl is 74.55 g/mol; number of moles = Mass/ Molar mass
So, the number of moles = 21.89 g ÷ 74.55 g/mol = 0.2936 mol
and the number of molecules = Number of moles * Avogadro's number
Number of molecules = 0.2936 mol x 6.02 x 10²³ molecules/mol
Number of molecules = 1.765 x 10²³ molecules
Therefore, there are approximately 1.765 x 10²³ molecules of KCl in 21.89 grams of KCl.
To know more about potassium chloride, refer
https://brainly.com/question/25380525
#SPJ1