What volume will a gas occupy at 325 K if it occupies 5.8 L at 376 K? Assume the moles and pressure of gas are held constant
A) 2.89 L
B) 3.76 L
C) 5.01 L
D) 6.73 L
Answers
Answer:
5.01 L
Explanation:
my guy i just took the test, it was this one
Related Questions
A container of hydrogen peroxide originally has a mass of 28.96 grams and then completely decomposes into water and oxygen gas. The mass of water produced is 15.33 g. What mass of oxygen gas was also produced?
Answers
Answer:
dhshrvehduvhdhehejieeiie
PLEASE ANSWER QUICK I NEED TO FINSH THIS!!!! 20 POINTS!!!
which choice identifies the correct limiting reactant and correct reasoning?
2H2 + O2 --> 2H2O
0.4g H2 produces 0.20 mol moles H2O 1.8g O2 produces 0.22 moles H2O
A.) O2 because it was higher yield
B.) H2 because it has the lower yield
C.) H2 because it has the lower starting mass
D.) O2 because it has the higher starting mass
Answers
The limiting reactant in the chemical reaction is O₂ because because the O₂ contains the higher starting mass. The correct option is D.
The chemical equation is as :
2H₂ + O₂ ---> 2H₂O
The mass of the H₂ = 0.4 g
The molar mass of the H₂ = 2 g/mol
The moles of the H₂ = mass / molar mass
The moles of the H₂ = 0.4 / 2
The moles of the H₂ = 0.2 mol
The mass of the O₂ = 1.8 g
The molar mass of the O₂ = 32 g/mol
The moles of the O₂ = mass / molar mass
The moles of the O₂ = 1.8 / 32
The moles of the O₂ = 0.056 mol
2 moles of H₂ react with 1 mol of O₂
0.056 mol of O₂ react with = 2 × 0.056 = 0.112 mol of H₂
The O₂ is the limiting reactant. The correct option is D.
To learn more about limiting reactant here
https://brainly.com/question/31897248
#SPJ1
In the right triangle shown, m\angle V = 60\degreem∠V=60°m, angle, V, equals, 60, degree and UV= 18UV=18U, V, equals, 18.How long is UWUWU, W? Choose 1 answer: Choose 1 answer:
Answers
Answer:
9√3
Explanation:
Given that :
UV = 18
Angle V = 60°
To obtain the measure of UW = v
Using trigonometry :
Sinθ = opposite / hypotenus
Sinθ = UW / 18
θ = 60°
sin60° = UW / 18
Sin 60° = √3/2
√3 /2 = UW / 18
UW = 18 * √3/2
UW = 9 * √3
UW = 9√3

answer this step by step pls
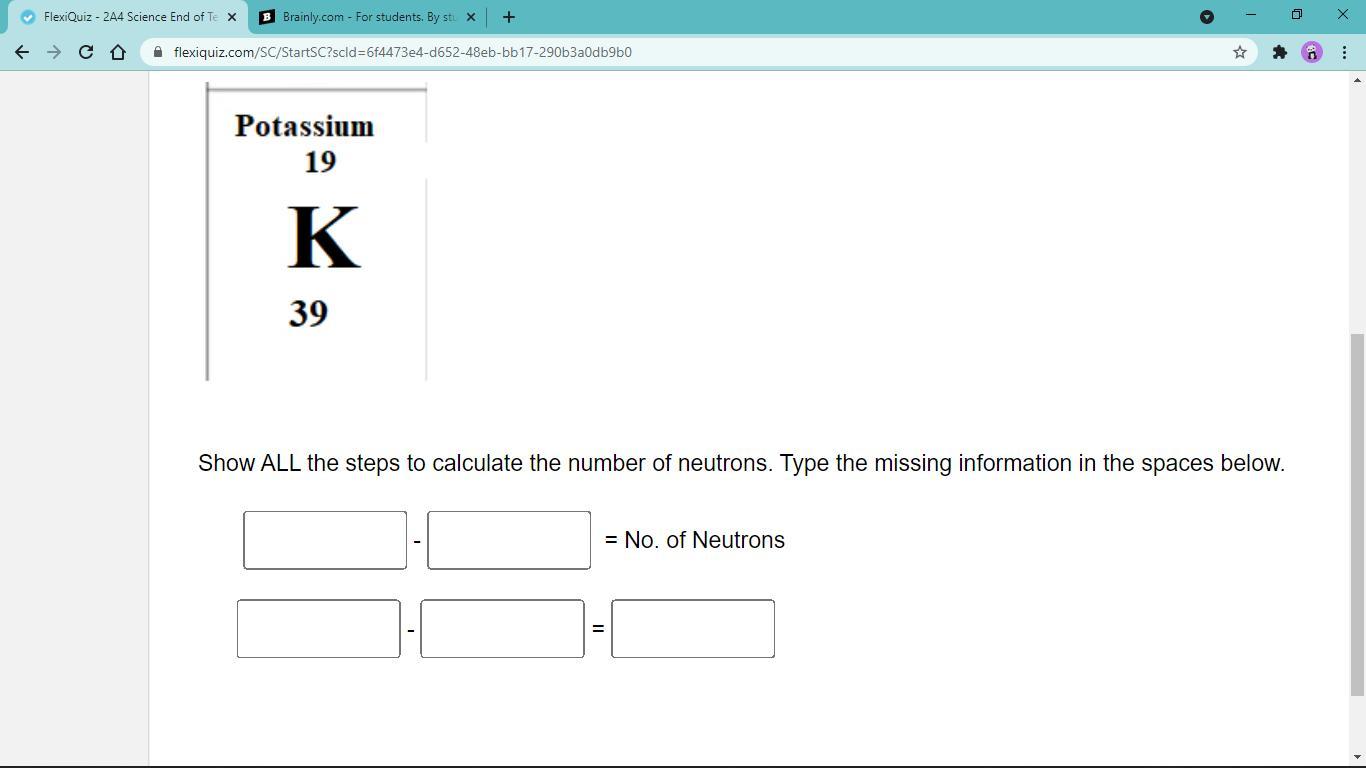
Answers
Answer:
neutrons = mass number - atomic number
. = 39-19 =20
for the following reaction find kp at 25°c and indicate whether kp should increase or decrease as the temperature rises. h2s(g) nh3(g) ⇌ nh4hs(s) δh° = - 83.47 kj and δg° = -17.5 kj at 25°c.
Answers
The value of Kp at 25°C for the reaction H2S(g) + NH3(g) ⇌ NH4HS(s) can be determined using the given values of ΔH° and ΔG°. Additionally, the effect of temperature on Kp can be determined by considering the sign of ΔH°.
The equilibrium constant Kp is related to the standard Gibbs free energy change ΔG° through the equation ΔG° = -RTln(Kp), where R is the gas constant and T is the temperature in Kelvin. Given that ΔG° is -17.5 kJ at 25°C, we can solve for ln(Kp).
Next, we need to determine the relationship between ΔG° and ΔH°. Since ΔG° = ΔH° - TΔS°, where ΔS° is the standard entropy change, we can rearrange the equation to obtain ΔH° = ΔG° + TΔS°.
Given that ΔH° is -83.47 kJ at 25°C, we can substitute the values into the equation and solve for ΔS°. Once ΔS° is determined, we can plug the values of ΔH° and ΔS° into the Van't Hoff equation ln(Kp2/Kp1) = ΔH°/R * (1/T1 - 1/T2),
where Kp1 and Kp2 are the equilibrium constants at temperatures T1 and T2, respectively.
By rearranging the Van't Hoff equation and solving for Kp2, we can calculate the value of Kp at a different temperature. Based on the sign of ΔH°, we can determine whether Kp will increase or decrease as the temperature rises.
Using this approach, we can calculate Kp at 25°C for the given reaction using the values of ΔH° and ΔG°. Additionally, we can determine the effect of temperature on Kp by considering the sign of ΔH°, indicating whether Kp will increase or decrease as the temperature rises.
Learn more about equilibrium constant Kp here:
https://brainly.com/question/30550192
#SPJ11
what is a microstate?select the correct answer below:the smallest number of particles that can occupy an energy level.the unique distribution of particles among energy levels.the number of particles in a mole of a substance.a way to measure the total energy in a system.
Answers
The correct answer is: The unique distribution of particles among energy levels.
A microstate refers to a specific arrangement or distribution of particles among the energy levels in a system.
In the context of statistical mechanics, a system is often composed of a large number of particles, such as atoms or molecules, each possessing certain energy levels.
A microstate describes the precise occupation of these energy levels by the particles at a given moment.
The concept of microstates helps us understand the behavior and properties of systems at the microscopic level.
By examining the various possible microstates and their corresponding probabilities, statistical mechanics provides insights into macroscopic observables, such as temperature and pressure.
The other options provided are incorrect. The smallest number of particles that can occupy an energy level is determined by the system's quantum properties, not by microstates.
The number of particles in a mole of a substance is determined by Avogadro's number.Microstates are not directly related to measuring the total energy in a system; they describe the arrangement of particles within energy levels, not the energy itself.
To know more about microstate refer here
brainly.com/question/13865331#
#SPJ11
If there are 0.505 g of NaCl left in a beaker that originally contained 75.0 mL of saltwater, what must have been the concentration of the original solution? a. 0.00647 M b. 0.0115 M c. 0.0673 M d. 0.115 M e. 0.673 M
Answers
If there are 0.505 g of NaCl left in a beaker that originally contained 75.0 mL of saltwater, what must have been the concentration of the original solution is 0.673 M.
The correct answer is option e. 0.673 M.
The concentration of the original solution, we need to use the formula: concentration = amount of solute / volume of solution. First, we need to convert the mass of NaCl to moles. The molar mass of NaCl is 58.44 g/mol.
0.505 g NaCl x (1 mol NaCl/58.44 g NaCl) = 0.00863 mol NaCl.
First, we need to find the number of moles of NaCl. To do this, we will use the molar mass of NaCl (58.44 g/mol). Moles of NaCl = mass (g) / molar mass (g/mol) = 0.505 g / 58.44 g/mol ≈ 0.00864 mol, 2. Next, we will convert the original volume of the solution from mL to L. 75.0 mL = 75.0 / 1000 L = 0.075 L, 3. Finally, we will find the concentration (molarity) of the original solution. Concentration (M) = moles of solute / volume of solution (L) = 0.00864 mol / 0.075 L ≈ 0.115 M
To know more about solution visit:
https://brainly.com/question/30665317
#SPJ11
what are the effects on people when fish fail to reproduce and become extinct
Answers
Answer: People will have less food to eat and less nutrition and the food chain will go down. Sorry if I'm not right.
Explanation:
Answer:
It will effect all our wildlife and waters Becuase some fishes eat alge and other animals that reporduce fast and it will break the food chain many animals will die
Explanation:
Can someone help with these

Answers
One strategy is to incorporate a soil bacterium gene that produces a glyphosate tolerant form of EPSPS.
Another way is to incorporate a different soil bacterium gene that produces a glyphosate degrading enzyme.
Suggest some changes that could be made to this experiment to obtain a more reliable or more precise value for the optimum temperature
Answers
To obtain a more reliable or precise value for the optimum temperature in an experiment, several changes can be made. Here are some suggestions: Replicate the experiment, Increase sample size, Use a narrower temperature range, Utilize more precise temperature control etc
Replicate the experiment: Conducting multiple repetitions of the experiment and calculating the average of the results can help reduce random errors and increase the reliability of the obtained value.Increase sample size: Using a larger sample size can enhance the precision of the data. This provides a more representative picture of the behavior at different temperatures, reducing the impact of outliers or random fluctuations.Use a narrower temperature range: Instead of testing a wide range of temperatures, focus on a narrower range around the expected optimum temperature. This allows for more precise measurements and a better understanding of the specific region where the optimum occurs.Utilize more precise temperature control: Ensure the temperature control apparatus or equipment used in the experiment is capable of maintaining a consistent and accurate temperature. Using advanced temperature control methods, such as precision thermostats or water baths, can minimize temperature fluctuations and improve the accuracy of measurements.Increase measurement frequency: Taking measurements at more frequent intervals during the temperature range can provide a more detailed profile of the response, allowing for a more precise determination of the optimum temperature.For more such questions on optimum temperature visit:
https://brainly.com/question/4735135
#SPJ8
How many moles are in 5.25 X 10 25
atoms of Au?
Answers
Answer:
The answer is 87.21 molesExplanation:
To find the number of moles in a substance given it's number of entities we use the formula
\(n = \frac{N}{L} \\ \)
where n is the number of moles
N is the number of entities
L is the Avogadro's constant which is
6.02 × 10²³ entities
From the question we have
\(n = \frac{5.25 \times {10}^{25} }{6.02 \times {10}^{23} } \\ = 87.2093023...\)
We have the final answer as
87.21 molesHope this helps you
to what fraction of its original volume, vfinal/vinitial, must a 0.40−mole sample of ideal gas be compressed at constant temperature for δssys to be −4.3 j/k?
Answers
The problem involves calculating the change in entropy (δS) of an ideal gas during a constant temperature compression process. We can use the formula δSsys = -nRln(Vfinal/Vinitial) to solve for the ratio of final volume to initial volume (Vfinal/Vinitial). Then, the gas needs to be compressed to 0.0000174 times its original volume to achieve a δSsys of -4.3 J/K.
To determine the fraction of its original volume that a 0.40-mole sample of ideal gas must be compressed at constant temperature for δssys to be -4.3 J/K, we can use the formula: δssys = -nR ln(vfinal/vinitial)
where n is the number of moles of gas, R is the gas constant, and vfinal/vinitial is the ratio of final volume to initial volume.
Rearranging this formula, we get:
vfinal/vinitial = e^(-δssys/nR)
Plugging in the given values, we have:
vfinal/vinitial = e^(-(-4.3)/(0.40 mol x 8.31 J/(mol K)))
vfinal/vinitial = e^(13.5)
vfinal/vinitial = 57387.4
Therefore, the 0.40-mole sample of ideal gas must be compressed to 1/57387.4 or about 0.0000174 times its original volume to achieve a δssys of -4.3 J/K at constant temperature.
To learn more about entropy; https://brainly.com/question/15022152
#SPJ11
The phenol group activates benzene ring for electrophilic aromatic substitution in the ortho (adjacent carbons) and para (opposite carbons) ring positions over substitution at the meta (two carbons away) positions. Identify the substituted phenol compound that has both ortho and meta substitution.
Answers
A substituted phenol compound that has both ortho and meta substitution is 2-nitrophenol.
What is phenol compound?Phenol (also known as carbolic acid) is a C6H5OH aromatic organic compound. It's a volatile white crystalline solid. A phenyl group (C6H5) is bonded to the a hydroxy group (OH) in the molecule. It is mildly acidic and must be handled with caution since it may cause chemical burns.
Phenol was originally extracted from coal tar but is now produced on either a large scale (approximately 7 billion kg/year) from oil feedstocks. As a forerunner to too many materials but instead useful compounds, it is a valuable industrial commodity.
The nitro group (-NO2) is an activating and ortho/para-directing substituent, which will direct the electrophile to the ortho and para positions. The nitro group is also a meta-directing substituent, which will direct the electrophile to the meta position.
To learn more about phenol compound
https://brainly.com/question/2437659
#SPJ4
Somebody claims to have developed a new reversible heat-engine cycle that has a higher theoretical efficiency than the Carnot cycle operating between the same temperature limits. How do you evaluate this claim?
Answers
Somebody claims to have developed a new reversible heat-engine cycle that has a higher theoretical efficiency than the Carnot cycle operating between the same temperature limits. This evaluates that this heat engine is less efficient.
The Carnot's cycle is an ideal cycle for all heat engine which operates between the same temperature. It is a reversible cycle which have all process reversible that is why it have maximum efficiency.
On the other hand, the new reversible heat engine is a practical working cycle so it is impossible to make all process reversible . Practically, there will be always loss of energy due to this any process can not be 100 % reversible. That is why the new reversible heat-engine cycle have low efficiency as compared to Carnot cycle operating between same temperature limits.
To know more about carnot cycle here
https://brainly.com/question/14943330
#SPJ4
the average molecular speed in a sample of gas at a certain temperature is 296 m/s. the average molecular speed in a sample of gas is m/s at the same temperature.
Answers
The average molecular speed in a sample of gas at a certain temperature is 296 m/s.
To find the average molecular speed in a sample of gas at the same temperature, we can use the formula for the root-mean-square (RMS) speed of gas molecules. The RMS speed is equal to the square root of (3RT/M), where R is the ideal gas constant, T is the temperature in Kelvin, and M is the molar mass of the gas.
Since the temperature is the same in both samples, the RMS speed will also be the same. Therefore, the average molecular speed in the second sample of gas at the same temperature is also 296 m/s.
To know more about molecular visit:-
https://brainly.com/question/156574
#SPJ11
Calculate the percent ionic, the percent covalent, and the bond length (in picometers) of a chemical bond between phosphorus and selenium.
Phosphorus—atomic radius: 109 pm; covalent radius: 106 pm; ionic radius: 212 pm.
Selenium—atomic radius: 122 pm; covalent radius: 116 pm; ionic radius: 198 pm.
98 percent ionic, 2 percent covalent, 410 pm
4 percent ionic, 96 percent covalent, 222 pm
2 percent ionic, 98 percent covalent, 222 pm
96 percent ionic, 4 percent covalent, 410 pm
Answers
Answer:
The correct option is;
4 percent ionic, 96 percent covalent, 222 pm
Explanation:
The parameters given are;
Phosphorus:
Atomic radius = 109 pm
Covalent radius = 106 pm
Ionic radius = 212 pm
Electronegativity of phosphorus = 2.19
Selenium:
Atomic radius = 122 pm
Covalent radius = 116 pm
Ionic radius = 198 pm
Electronegativity of selenium= 2.55
The percentage ionic character of the chemical bond between phosphorus and selenium is given by the relation;
Using Pauling's alternative electronegativity difference method, we have;
\(\% \, Ionic \ Character = \left [18\times (\bigtriangleup E.N.)^{1.4} \right ] \%\)
Where:
Δ E.N. = Change in electronegativity = 2.55 - 2.19 = 0.36
Therefore;
\(\% \, Ionic \ Character = \left [18\times (0.36)^{1.4} \right ] \% = 4.3 \%\)
Hence the percentage ionic character = 4.3% ≈ 4%
the percentage covalent character = (100 - 4.3)% = 95.7% ≈ 96%
The bond length for the covalent bond is found adding the covalent radii of both atoms as follows;
The bond length for the covalent bond = 106 pm + 116 pm = 222 pm.
The correct option is therefore, 4 percent ionic, 96 percent covalent, 222 pm.
Complete the following oxidation-reaction:(3 points) 1+ CH2 1H 3. Label each of the following structures as a hemiacetal, hemiketal, acetal, ketal or none of these. (3 points) a. b. O CH2CH3 OCH CH3 CHCH-OH d. 6.Match the structural formula with the correct functional group: (4 points) A) aldehyde a CH3CH,OH B) ether C) ketone e. CH3OH D) alcohol
Answers
Complete the following oxidation-reaction: 1+ CH2 1H 3. The oxidation-reaction is CH2 + 1H3 --> CH3 + H2O.
Label each of the following structures as a hemiacetal, hemiketal, acetal, ketal or none of these.
A) Hemiacetal - CH2CH3OCH2CH3
B) Acetal - OCH2CH3
C) Hemiketal - CH2CHOH
D) None of these - CH3OH
Match the structural formula with the correct functional group:
A) Aldehyde - CH3CHO
B) Ether - CH3OCH3
C) Ketone - CH3COCH3
D) Alcohol - CH3OH
To know more about oxidation reaction refer to-
brainly.com/question/19528268#
#SPJ11
to what volume in ml should you dilute 100ml of a 2.50 m CaCl2 solucion to obtain 0.75 M CaCl2 solution
Answers
GIVEN :
• Volume ,(V1)= 100ml
• Concentration ,(C1) = 2.50 M
• Concentration ,(C2) = 0.75 M
• Volume ,(V2) = ?
The relationship between Volume and Concentration can be represented by formula :
\(C_{1\text{ }}V_1=C_2V_2\)Replacing the given parameters into the formula above , Final Volume (V2) will be :
\(\begin{gathered} C_1V_1=C_2V_2 \\ \therefore V_{2\text{ }}=\text{ }\frac{C_1\cdot V_1}{C_2} \\ \text{ =}\frac{2.50\text{ M }\cdot100\text{ ml}}{0.75\text{ M }} \\ \text{ = }333.33\text{ ml} \end{gathered}\)This means thea final volume = 333.33mlWhat is the difference between a compound light and an electron microscope?
Answers
Answer:
Electron microscopes differ from light microscopes in that they produce an image of a specimen by using a beam of electrons rather than a beam of light. Electrons have much a shorter wavelength than visible light, and this allows electron microscopes to produce higher-resolution images than standard light microscopes.
Explanation:
cheakIf either allele is the allele for a trait, the organism will exhibit that trait. An organism will express the recessive trait only when recessive
Answers
If either allele is a dominant allele for a trait, the organism will exhibit that trait. An organism will express a recessive trait only when both alleles are recessive.
What are alleles?Alternative gene forms are known as alleles. On a certain chromosome, it is situated at a specific position. Sexual reproduction is the means by which the alleles are transmitted from parents to children. These genes are a class of genetic material that codes for a particular characteristic of an organism.
An area on a chromosome known as a gene locus is where each allele is located. The two copies of the gene, one inherited from each parent, are found in the same area on two homologous chromosomes. Dominant or recessive alleles are both possible.
To learn more about allele, visit:
https://brainly.com/question/14756352
#SPJ1
The complete question is:
If either allele is the allele for a trait, the organism will exhibit that trait. An organism will express the recessive trait only when are recessive
6. a sample of nitrogen gas occupies 28.5 l at stp. how many moles of nitrogen are present?
Answers
Answer:
1 mol of N2
Mole of N2= 28.5 L × -------------------
22.4 L of N2
=1.272 mole of N2
DENSITY CHEMISTRY WORD PROB I WILL MARK BRAINLIEST PLEASE HELP I NEED ASAP

Answers
Answer:
The number of passengers that the Embraer E170 could carry if its fuselage was made of steel is 39 passengers
Explanation:
The given parameters of the cylinder representing the fuselage are;
The length of the cylinder, l = 30.0 meters
The width of the cylinder, w = 2.5 meters
The thickness of the cylinder, t = 2.5 mm = 0.0025 meters
The volume of the metal required to make the fuselage cylinder, V = (π·w²/4 - π·(w - 2·t)²/4) × l
Therefore, by substituting the known values, we have;
V = (π × 2.5²/4 - π×(2.5 - 2×0.0025)²/4) × 30 = 0.58845957392
The volume of the metal required to make the fuselage cylinder, V≈ 0.58846m³
The given density of steel = 7.87 g/cm³ = 7870 kg/m³
The given density of aluminum = 2.70 g/cm³ = 2,700 kg/m³
Mass = Density × Volume
The mass of the fuselage made with steel = The given density of steel × The volume of the metal required to make the fuselage cylinder
∴ The mass of the fuselage made with steel = 7870 × 0.58846 ≈ 4631.18
The mass of the fuselage made with steel ≈ 4631.18 kg
Similarly, the mass of the fuselage made with aluminum = 2,700 × 0.58846 ≈ 1588.84
The mass of the fuselage made with aluminum ≈ 1588.84 kg
The total mass the Embraer E170 could carry = The mass of the fuselage made with aluminum + The mass of the 78 passengers
The average mass of each passenger = 79 kg
∴ The total mass the Embraer E170 could carry ≈ 1588.84 + 78 × 79 = 7750.84
The total mass the Embraer E170 could carry ≈ 7750.84 kg
The extra mass the Embraer E170 could carry when made of steel = (The total mass the Embraer E170 can carry) - (The mass of the fuselage made with steel)
∴ The extra mass the Embraer E170 could carry when made of steel = 7750.84 - 4631.18 = 3119.36
The extra mass the Embraer E170 could carry when made of steel ≈ 3119.36 kg
The number of passengers the Embraer E170 could carry when made of steel = (The extra mass the Embraer E170 could carry when made of steel)/(The average mass of one passenger)
∴ The number of passengers the Embraer E170 could carry if its fuselage was made of steel = 3119.36 kg/(79 kg/passenger) ≈ 39.489 passengers
The number of passengers that the Embraer E170 could carry if its fuselage was made of steel ≈ 39 passengers (we round down when counting people which are whole quantities)
100 POINTS PLS HELP!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!

Answers
Answer:
i need this im struggling with my work
Explanation:
Last one and the first
PLEASE HELP!! Due in a few minutes!!! BRAINLEST TO WHO ANSWERS FIRST!!!
2.2.4 Study: Reaction Rates
POSSIBLE POINTS: 1.
Collisions between reactant molecules lead to a reaction when the molecules have the correct orientation and enough kinetic energy
True
False
Answers
Answer:
True
Explanation:
Hope this isnt too late
Explain the effect of carbon dioxide on the ph of the oceans.
Answers
Answer:
When carbon dioxide dissolves in seawater, the water becomes more acidic and the ocean's pH (a measure of how acidic or basic the ocean is) drops.
Explanation:
hope this helps
2. Consider dimethyl ether at 300 K which has an angle averaged radius of 0.25 nm. a) Calculate its collision frequency at 1 bar and 1 Pa. b) Calculate its decomposition rate constant k (CH3)2CO produ
Answers
a) The collision frequency of dimethyl ether can be calculated using the kinetic theory of gases. The collision frequency is given by the equation:
\(\[\text{{Collision frequency}} = \frac{1}{4} \sqrt{\frac{8 \cdot k \cdot T}{\pi \cdot m}}\]\)
where k is the Boltzmann constant, T is the temperature in Kelvin, and m is the mass of dimethyl ether molecule. Given that the angle-averaged radius of dimethyl ether is 0.25 nm, we can calculate the mass of the molecule using its density or molar mass.
b) To calculate the decomposition rate constant of (CH3)2CO, we need additional information such as the reaction mechanism and reaction conditions. The rate constant for a chemical reaction depends on factors like temperature, activation energy, and the presence of catalysts. Without these details, it is not possible to calculate the decomposition rate constant accurately.
In conclusion, the collision frequency of dimethyl ether at a specific temperature can be calculated using the kinetic theory of gases. However, to calculate the decomposition rate constant of (CH3)2CO, additional information about the reaction conditions and mechanism is needed.
To know more about Molar Mass visit-
brainly.com/question/31545539
#SPJ11
Identifying Physical and Chemical Properties
Answers
Ques: how to identify physical or chemical properties
Ans: you can identify them by their properties. make sure that it matches its property. but you must know the physical and chemical properties . i will give an example .so how to identify boiling of eggs and changing of water into ice. Physical properties
-it can be reversed back
-it is temporary change
-no new substance is formed
chemical properties
-it cannot be reversed back
-it is permanent change
-new substance is formed
now boiling of eggs matches all property of chemical change so it is chemical change. changing of water to ice matches all property of physical change so it is physical change.
hope it helps
Need help with 6th grade science homework PLZ HELP ASAP
Answers
Answer:
whaa is it
Explanation:
Answer:
There's nothing. What do you need help with?
Explanation:
Can someone pleaseeee help if you’re correct I’ll give u brainlist

Answers
Answer:
I think it the second one adding external weight or the third one
Explanation:
I could never. im more like the last one
I'm really sorry if this was no help
What is the density of a 700 kg object with a volume of 649 m'? (Density: D™)
0.927 kg/m
3.4543 kg/m
1.079 kg/m
4.543 kg/m
Answers
Answer: 1.079 kg/m3
Explanation:
The formula for the density is as follows as; Here, D is the density, m is the mass and V is the volume. Density is inversely proportional to the volume and it is directly proportional to the mass. It is given in the problem that density of an object is 700 kg having volume .
d=m/V or p=m/V were d and p are the same thing d stands for density and p is the greek symbol rho so d=p they are the same thing. m stands for mass and V stands for volume
density = mass/Volume
d=700kg/649^3
=1.079 kg/m^3
( Don’t forget (^3) power of 3 because is important for the volume, for ex. m^3 cm^3 km^3 etc)
The answer is 1.079 kg/m^3