Where is DNA found?
a. cell membrane
b. vacuole
c. chromosomes
d. Golgi body
Answers
Answer:
You get DNA from your cell membrane
Explanation:
Related Questions
Which pair of objects is experiencing the greatest gravitational force
Answers
Answer:
Explanation:
Is that the question
Answer:
If it's between human and tennis ball, human and dog, human and building, human and earth.
It would be HUMAN AND EARTH
Explanation:
I took the test and got that.
how many electrons are in bromine’s (atomic number 35) next to outer shell (n=3)?
Answers
shell3=2×3^2=18
shell one =2
shell 2=8
2+8+18=28
28 electron all together one the 3rd shell
35-28=7
7 electrons on the 4th shell
In bromine's n=4 shell, we have a a total of 2 + 5 = 7 electrons.
How do we know?In bromine's (atomic number 35) electron configuration, the next outer shell after the third shell (n=3) is the fourth shell (n=4).
We will subtract the total number of electrons in the previous shells, in order to determine the number of electrons in the n=4 shell
The electron configuration of bromine (Br) is:
\(1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5\)
We then count the electrons in the n=4 shell, we consider the electrons in the 4s and 4p subshells.
In the 4s subshell, we have 2 electrons (\(4s^2\)).
In the 4p subshell, there are 5 electrons (\(4p^5\)).
Learn more about electron configuration at:
https://brainly.com/question/26084288
#SPJ4
3. How many grams of NaOH are produced if 20.0 g of sodium metal react with excess oxygen?
2Na(s) + 2H₂O(l) →→2NaOH(aq) + H₂(g)
Pls answer with explanation

Answers
The amount of NaOH produced when 20.0 g of Na(s) reacts with excess oxygen is 38.4 grams.
Stoichiometric problemTo find the amount of NaOH produced when 20.0 g of Na reacts, we first need to convert the mass of Na to moles using the molar mass of Na, which is 22.99 g/mol.
Number of moles of Na = 20.0 g / 22.99 g/mol = 0.870 mol
Since the balanced equation shows that 2 moles of Na react to produce 2 moles of NaOH, we can use stoichiometry to find the number of moles of NaOH produced.
Number of moles of NaOH = 0.870 mol Na x (2 mol NaOH/2 mol Na) = 0.870 mol NaOH
Now, we can convert the number of moles of NaOH to grams using the molar mass of NaOH, which is 40.00 g/mol.
Mass of NaOH produced = 0.870 mol NaOH x 40.00 g/mol = 34.8 g
Therefore, 34.8 grams of NaOH are produced when 20.0 g of Na(s) react with excess oxygen.
More on stoichiometric problems can be found here: https://brainly.com/question/28297916
#SPJ1
What would happen to the density of an object if you have a larger piece of it? (Example: comparing a boulder to a pebble.)
Answers
The density of that substance will always be the same. if you cut the object into a million pieces, they would still each have the same density.
What happens to the density if you have a larger piece of it?Because a material that dilates takes up a larger volume, its density decreases. This occurrence occurs in pieces in all forms of matter: for example, solids, liquids, and gases. The size, mass, and disposition of atoms affect the density of a substance.
The density of an object can convert if either the mass or volume of the object is changed. This means that the density of the general object will decrease and be more likely to float.
So we can conclude that The density of a liquid is a computation of how heavy it is for the amount measured.
Learn more about density here: https://brainly.com/question/1354972
#SPJ1
6) Which of the following is the highest pressure?
A. 1000 atm
b. 1000 mmHg
C. 1000 torr
Answers
Ascorbic acid has a molar mass of 176. 14 g/mol. What is the molecular formula of ascorbic acid?
Answers
The molecular formula of Ascorbic acid is C6H8O6 having the molar mass of 176.14 g/ mole.
Ascorbic acid is known as the chemical name for Vitamin C. Ascorbic acid is commonly found in high concentrations in citrus fruit. This acid is also found in tomatoes, broccoli, and many other fruits and vegetables. Vitamin C is a nutrient of the body needs to form blood vessels, cartilage, muscle and collagen in bones. This vitamin is also vital to the body's healing process.
A molecular formula is defined as a chemical formula of a molecular compound that shows the kinds and numbers of atoms present in a molecule of the compound. This is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule using chemical element symbols and numbers.
A molecule of the ascorbic acid will have a mass of 176.124 atomic mass units.
This is determined by adding 6 X 12.011 for carbon + 8 X 1.008 for hydrogen + 6 X 15.999 for oxygen.
This is equals to the 72.066 for carbon + 8.064 for hydrogen + 95.994 for oxygen. Added together, these equal 176.124 molecular mass.
To learn more about Ascorbic acid
https://brainly.com/question/16996726
#SPJ4
The final electron acceptor in aerobic respiration is....... NAD water oxygen pyruvate O hydrogen . Answer al Question 16 Which of the following processes generate carbon dioxide? Hint There are more than one. Glycolysis Oxidative Phosphorylation The Link Reaction (pyruvate oxidation) The Citric Arid Cycle Lactic Acid Fermentation Alcoholic Fermentation
Answers
The final electron acceptor in aerobic respiration is oxygen (O2).The electron transport chain (ETC) in cellular respiration relies on a final electron acceptor to help oxygen get reduced into water. This is why oxygen is considered the final electron acceptor in cellular respiration.
During cellular respiration, glucose is broken down into pyruvate. Pyruvate is then transformed into acetyl CoA and enters the citric acid cycle, where it is oxidized and generates ATP, NADH, and FADH2. The final stage of aerobic respiration involves the electron transport chain, in which electrons from NADH and FADH2 are passed through a series of proteins and coenzymes in the inner mitochondrial membrane, ultimately reducing oxygen to form water.
This process is known as oxidative phosphorylation.In conclusion, the final electron acceptor in aerobic respiration is oxygen (O2), and carbon dioxide is generated in the link reaction (pyruvate oxidation) and the citric acid cycle.
To know more about cellular respiration visit:-
https://brainly.com/question/32872970
#SPJ11
When carbon dioxide enters the blood, some of it combines with ____________ to form carbaminohemoglobin, but most of it becomes ____________ ions that diffuse into the blood plasma.
Answers
Answer:
hemoglobin to become bicarbonate
Explanation:
When carbon dioxide enters the blood it combines with hemoglobin to form carbamino hemoglobin or carboxyhemoglobin but most of it becomes carbonate ions and diffuse into the blood plasma.
What is carboxyhemoglobin?Hemoglobin is a blood protein giving red colour to the blood. It helps to transport oxygen everywhere. When oxygen fills in the air sack, it combines with oxygen and form oxyhemoglobin.
However, hemoglobin have more affinity towards carbon dioxide and and they combines to form carbaminohemoglobin which prevents the transportation of oxygen through blood.
The carbon dioxide will be converted into carbonic acid by combining with water. This acid then forms its carbonate ions and these ions helps to maintain the blood pH to be constant.The carbonate ions, CO₃²⁻can easily diffuse into blood plasma.
To find more about carboxyhemoglobin, refer the link:
https://brainly.com/question/13056928
#SPJ5
A 50-gallon drum is being used to concentrate clean water that is flowing into the top of the drum with chlorine.
Water flows in at a rate of 5 gallons per hour, with chlorine concentrated at 3 grams per gallon. Chlorinated water is then being
pumped out at the same rate to keep the drum full of liquid without overflowing. The water is initially clean and contains no
chlorine.
a) Write a differential equation modeling the rate at which chlorine accumulates in the drum, y, in grams, t hours since the concentration process begins.
b) Find any equilibrium point(s) and explain the practical meaning of this value(s).
c) Using the idea of a phase line (do not solve the ODE), describe what we can expect to happen to the amount of chlorine in the tank in the long-run.
Answers
A) The differential equation is dy/dt = (3/50) * 5 - (y/50) * 5.
B) Equilibrium point: y = 30g. It represents the steady-state chlorine concentration where the inflow rate matches the outflow rate.
C) The chlorine amount will approach and stabilize at 30g. No net change occurs as the inflow matches the outflow.
In part A, we are given the information about the rate at which clean water with chlorine is flowing into the drum and being pumped out to maintain the liquid level. The differential equation dy/dt = (3/50) * 5 - (y/50) * 5 models the rate at which chlorine accumulates in the drum over time. The first term represents the inflow rate of chlorine, and the second term represents the outflow rate. By subtracting the outflow rate from the inflow rate, we get the net rate of accumulation of chlorine in the drum.
In part B, we find the equilibrium point(s) by setting the rate of accumulation (dy/dt) to zero and solving for y. The equation (3/50) * 5 - (y/50) * 5 = 0 simplifies to 3 - y/10 = 0, and solving this equation gives y = 30. This means that when the concentration of chlorine in the drum reaches 30 grams, the inflow rate of chlorine matches the outflow rate, resulting in a steady-state concentration.
The practical meaning of this equilibrium value is that the drum will maintain a constant chlorine concentration of 30 grams in the long run, as long as the inflow and outflow rates remain unchanged.
In part C, using the concept of a phase line, we can expect that the amount of chlorine in the tank will approach and stabilize at the equilibrium value of 30 grams in the long run. Since the inflow rate of chlorine is balanced by the outflow rate, there will be no net change in the concentration over time. The system will reach a stable state where the chlorine concentration remains constant. Thus, the chlorine amount will remain at 30 grams indefinitely.
Learn more about differential equation
brainly.com/question/33433874
#SPJ11
What is the volume of kristas rock

Answers
Answer : The volume of kristas rock is 30 mL.
Explanation :
From the given image we conclude that:
Initial volume of liquid = 150 mL
Final volume of liquid = 180 mL
Now we have to determine the volume of kristas rock.
Volume of kristas rock = Final volume of liquid - Initial volume of liquid
Volume of kristas rock = 180 mL - 150 mL
Volume of kristas rock = 30 mL
Therefore, the volume of kristas rock is 30 mL.
HELPPP PLEASE TIME LIMITED!!!

Answers
A particular oxidoreductase requires FAD as an essential component for its activity. The complete biological unit required for reaction is called:
Answers
The complete biological unit required for the reaction is called a holoenzyme.
A holoenzyme is an active enzyme consisting of both the protein part (called the apoenzyme) and the non-protein components, which can be cofactors or coenzymes.
In this case, the particular oxidoreductase requires FAD (flavin adenine dinucleotide) as an essential component for its activity. FAD serves as a coenzyme, helping the enzyme to carry out its function by facilitating the redox reaction.
These proteins are involved in a variety of cellular processes, including oxidation-reduction reactions, electron transport, and metabolism.
To sum up, the complete biological unit required for the reaction involving the particular oxidoreductase and FAD is known as a holoenzyme, which consists of the apoenzyme and the coenzyme FAD.
For more information on holoenzyme kindly visit to
https://brainly.com/question/30517781
#SPJ11
determine how much heat (in kj) of 2.89 mol of tio2(s)
Answers
Total heat generated by 2 mole of TiO2(s) is 4.963kJ.
The amount of heat released in the reaction of 2.89 mol of TiO2(s) can be calculated using the following equation: q = nCΔT, where n is the number of moles, C is the specific heat capacity of TiO2, and ΔT is the change in temperature.
The specific heat capacity of TiO2 is 683. 697. J/kgK. and the change in temperature is is 25k. By plugging in the values and converting J to kJ,
q = 2.89 * 25 * 683.697
=> 4963.35
In brief, the amount of heat released by 2.89 mol of TiO2(s) is 4.963kJ.
To know more about specific heat capacity click on below link:
https://brainly.com/question/16952828#
#SPJ11
Complete question :
determine how much heat (in kj) of 2.89 mol of tio2(s) with a temperature difference of 25k
2 Ni(s) + 3 Br2(s)----> 2 NiBr3(s)
a. What has been oxidized?
b. What has been reduced
c. Qhat is the oxidizing agent?
d. What is the reducing agent
Answers
In the given reaction, nickel (Ni) has been oxidized while bromine (Br2) has been reduced.
In the given reaction, nickel (Ni) has been oxidized while bromine (Br2) has been reduced because nickel has lost electrons while bromine has gained electrons.
The oxidizing agent in the reaction is bromine (Br2) because it has gained electrons, which means it has undergone reduction. Bromine has a higher electronegativity than nickel, which allows it to pull electrons away from nickel and cause it to undergo oxidation.
The reducing agent in the reaction is nickel (Ni) because it has lost electrons, which means it has undergone oxidation. Nickel has a lower electronegativity than bromine, which makes it more likely to lose electrons and undergo oxidation.
Overall, the reaction represents a redox reaction, where one species (nickel) loses electrons and undergoes oxidation while the other species (bromine) gains electrons and undergoes reduction. This is an important process in many chemical reactions, including combustion, rusting, and many biological processes.
Know more about Combustion here:
https://brainly.com/question/15117038
#SPJ11
What information does the rate constant give from the rate law? A. It tells how much the rate of the reaction is affected by volume. B. It tells how much the rate of the reaction is affected by temperature. C. It tells how much the reaction rate is affected by activation energy. D. It tells how much the reaction rate is affected by concentrations. SUBMIT
Answers
From the rate law, the information that is given by the rate constant is: C. It tells how much the reaction rate is affected by activation energy.
What is the rate law?Rate law is also referred to as rate equation and it can be defined as a chemical equation that is typically used to relate the initial (forward) chemical reaction rate with respect to the concentrations or pressures of the chemical reactants and constant parameters.
Mathematically, the rate law is given by this formula:
\(R = k[A]^x[B]^y\)
Where:
k is the rate constant.A is the concentration of reactant A.B is the concentration of reactant B.In Chemistry, the rate constant is typically used to tell the reaction rate or rate of a chemical reaction that is affected by activation energy.
Read more on rate constant here: https://brainly.com/question/24749252
How do you multiply this or convert the units?
800cm x 6.45 s =
Answers
800cm x 6.45 s =51600 cm s will be the conversion value after multiplying .
What do you mean by the conversion of units ?Unit conversion is a multi-step process that involves multiplication or division by a numerical factor to convert one unit into another form of unit .
Uses of conversion of unit -:
The purpose of converting units is to obtain consistent correct answers.It is used to change the units of a measured quantity without changing its value.Without unit the values we obtain that are written are considered to be incomplete .51600 cm s is the conversion value obtained after converting the units .
Learn more about conversion of units ,here:
https://brainly.com/question/11543684
#SPJ1
pts) a. what would be the expected result if you added concentrated nitric acid (hno3) instead of hydrochloric acid to each test tube in step 7?
Answers
If we added concentrated nitric acid (hno3) instead of hydrochloric acid to each test tube in step 7 Both NaCl(s) and NaC,H,O,(s) would precipitate out of solution.
Salt is another name for sodium chloride. It occurs in sea and inland waters. Additionally, rock salt is obtainable. Seawater contains 1% to 5% sodium chloride. It is a crystalline, white substance. When it is in an aqueous state, it is referred to as a saline solution. This substance is water soluble and contains sodium cation and chloride anion. Sodium and chloride ions are present in a 1:1 ratio. It is commonly known as table salt and is primarily used in the food industry for flavouring and preservation. The pH of sodium chloride is 7.
To know more about Sodium Chloride visit : brainly.com/question/14516846
#SPJ4
how many moles of fe2 o3 are formed when 16.7 g of fe reacts completely with oxygen is available?
Answers
0.1495 moles of Fe2O3 are formed when 16.7 g of Fe reacts completely with oxygen. The calculation is based on the balanced chemical equation.
The substance condition for the response among Fe and O2 is:
4Fe + 3O2 → 2Fe2O3
This condition lets us know that 4 moles of Fe respond with 3 moles of O2 to create 2 moles of Fe2O3. In this manner, the mole proportion of Fe to Fe2O3 is 4:2 or 2:1.
To work out the quantity of moles of Fe2O3 framed when 16.7 g of Fe responds totally with oxygen, we first need to change the given mass of Fe over completely to moles utilizing its molar mass:
molar mass of Fe = 55.845 g/mol
moles of Fe = 16.7 g/55.845 g/mol = 0.299 mol
Since the mole proportion of Fe to Fe2O3 is 2:1, the quantity of moles of Fe2O3 framed is a portion of the quantity of moles of Fe that responded:
moles of Fe2O3 = 0.299 mol/2 = 0.1495 mol
In this way, 0.1495 moles of Fe2O3 are framed when 16.7 g of Fe responds totally with oxygen. This computation shows that stoichiometry, the connection between the amounts of reactants and items in a substance response, is significant in deciding how much item framed.
To learn more about fe reacts completely with oxygen, refer:
https://brainly.com/question/15658954
#SPJ4
A sentence using the term pseudoscience
Answers
Answer:
His phraseology and his turns of invention are too empirically pseudoscientific for the simplicity of nature.
How can we differentiate between sodium bromide and sodium iodide
Answers
Answer:
Sodium bromide is a chemical combination of bromine and iodine while sodium iodide is made up of sodium and iodine,you can also look at the difference in terms of boiling and melting poins
HF or HCl which can form the hydrogen bond? Explain your answer.
Answers
Answer:
can only form one hydrogen bond each
Explanation:
can only form one hydrogen bond each
how many grams of hydrogen are found in two moles of water, H2O?
Answers
4.032 g of hydrogen are found in two moles of water.
In order to determine how many grams of hydrogen are found in two moles of water (H2O), we must use the following equation:
1 mole of H2O = 2 moles of hydrogen atoms = 2(1.008 g/mol) = 2.016 g of hydrogen.
Therefore, two moles of water, H2O, would contain 4.032 g of hydrogen.
The molecular formula of water is H2O, meaning there are two hydrogen atoms for every one oxygen atom.
The molar mass of hydrogen is 2.016, so for every mole of water, there are two moles of hydrogen.
By multiplying two moles of hydrogen (2 x 2.016) by the number of moles of water (2), the total amount of hydrogen in two moles of water is 4.032 grams.
To learn more about water, click here:
https://brainly.com/question/28465561
#SPJ4
what are the factors affecting gravity?
Answers
Gravity, as a fundamental force of nature, is influenced by several factors. The following are some of the key factors affecting gravity:
Mass: The most significant factor affecting gravity is the mass of the objects involved. According to Newton's law of universal gravitation, the gravitational force between two objects is directly proportional to the product of their masses. Greater mass leads to a stronger gravitational force.Distance: The distance between two objects also plays a crucial role in the strength of gravity. According to the inverse square law, the gravitational force decreases as the distance between objects increases. As objects move farther apart, the gravitational attraction between them weakens.Gravitational Constant: The gravitational constant, denoted by G, is a fundamental constant in physics that determines the strength of the gravitational force. It is a universal constant and does not change, affecting the overall magnitude of gravity.Shape and Distribution of Mass: The distribution of mass within an object can influence the gravitational field it generates. Objects with a more compact and concentrated mass distribution will have a stronger gravitational pull compared to those with a more spread-out mass distribution.External Influences: Gravity can be influenced by external factors such as nearby celestial bodies or the presence of other forces. For example, the gravitational interaction between the Earth and the Moon affects tides on Earth's surface.In pasteur’s experiment, what would have happened if pasteur had broken off the swan neck of the flask one week after boiling the nutrient broth to sterilize the flask?.
Answers
Cells from the air would have fallen into the sterilized growth medium in the flask and grown there if Pasteur had broken off the swan neck of the flask one week after boiling the nutrient broth to sterilize the flask.
Who was Pasteur?He was referred to as a microbiologist who was responsible for many advances in science through experiments and an example is the one which was done using broth.
He heated the flask after it was sealed to kill microbes present in it and the result was nothing grew in the sample. However, if he had broken off the swan neck of the flask one week after boiling, cells from the air would have fallen into the sterilized growth medium in the flask and grown there.
Read more about Pasteur here https://brainly.com/question/11137072
#SPJ1
Cis- and trans-2-butene can both be hydrogenated to butane; thus their energies can be compared. the _____-isomer releases less energy upon hydrogenation therefore _____-2-butene higher in energy.
Answers
Cis- and trans-2-butene can both be hydrogenated to butane; thus their energies can be compared. the trans isomer releases less energy upon hydrogenation therefore cis-2-butene higher in energy.
What is hydrogenation?The process of addition of hydrogen atom to another compound in the presence of catalyst such as nickel, cobalt etc. is termed as hydrogenation process.
Why trans has lesser energy of hydrogenation?As in trans- isomer, similar atoms or group of atoms are on opposite side. Due to which there is less repulsion between the atoms which results in more stability of trans isomer. On the other hand in cis- isomer, similar atoms or group of atoms are on same side, due to which there is more repulsion between the atoms which results in less stability of cis-isomer.
Due to more stability of trans- isomer less energy is released to add hydrogen to the trans butene as compared to cis butene for the formation of butane.
Thus we concluded that the trans isomer releases less energy than cis isomer in hydrogenation process.
learn more about hydrogenation:
https://brainly.com/question/10150087
#SPJ4
What is the windward side of the sand dune

Answers
Answer:
A dunes windward side is the side where the wind is blowing and pushing material up. A dunes slip face is simply the side without wind. A slipface is usually smoother than a dunes windward side. A collection of dunes is called a dune belt or dune field.
Explanation:
Hope this helps...
classify the following compounds as ionic or covalent: bao, fe₂o₃, zno.
Answers
The compounds BaO, Fe₂O₃, ZnO, they all are ionic compounds.
Ionic compounds are compounds made up of positive and negative ions. Electrostatic forces hold them together. The following elements commonly form ionic compounds: nonmetals and metals, polyatomic ions, and transition metals.
Covalent compounds are compounds made up of two or more non-metallic elements. They join together by sharing electrons to achieve a stable electron configuration
BaO: Ba is a metal, while O is a nonmetal, therefore it's an ionic compound
Fe2O3: Fe is a metal, while O is a nonmetal, therefore it's an ionic compound
ZnO: Zn is a metal, while O is a nonmetal, therefore it's an ionic compound
Thus, all of the above compounds i.e. BaO, Fe₂O₃, ZnO are ionic compounds.
To learn more about ions :
https://brainly.com/question/13692734
#SPJ11
PLS HELP QUESTION 5 it’s due today and I already have a bad grade in her class
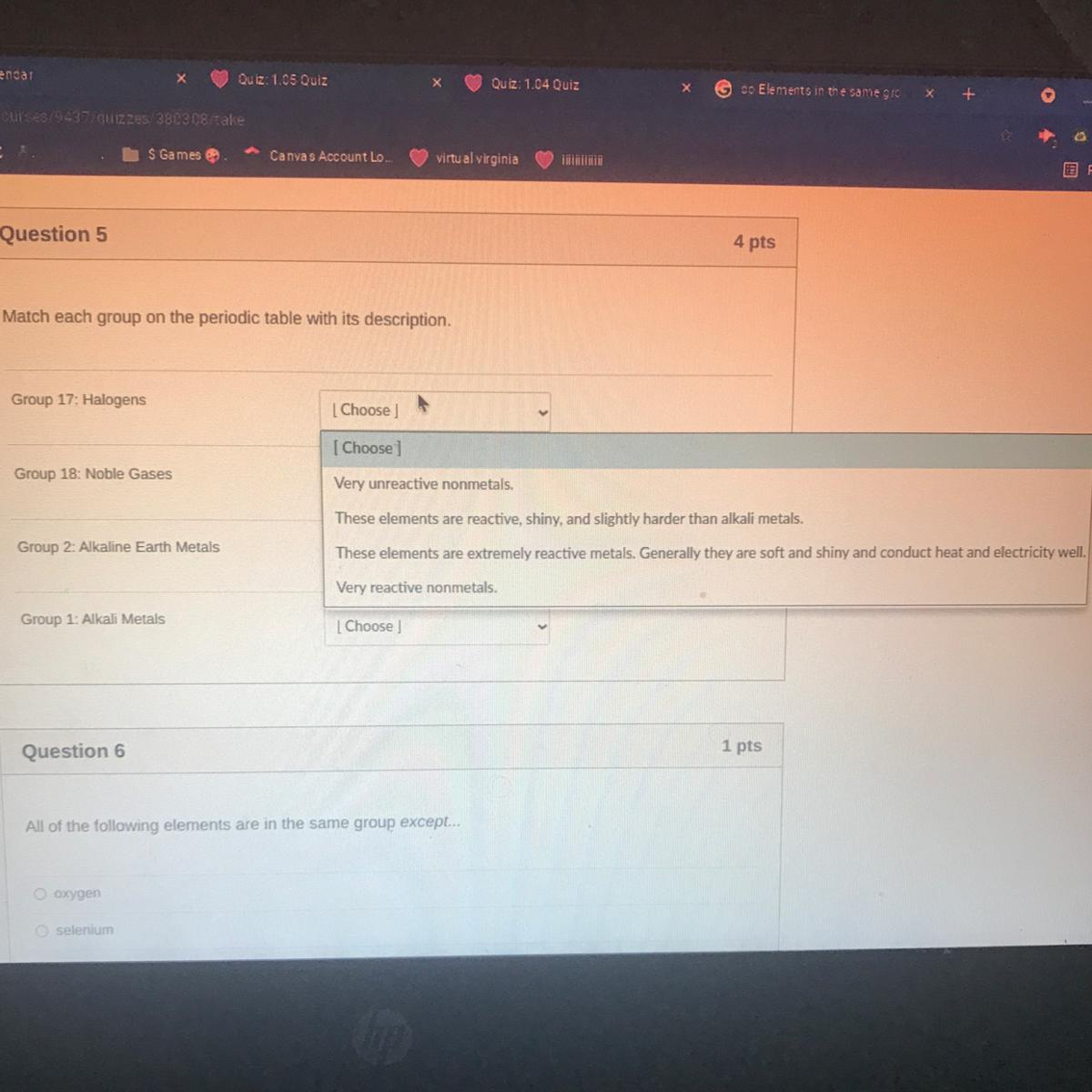
Answers
Answer:
very reactive non metals
Explanation:
keep working on her class
Answer:
very reactive non metals
Explanation:
Hope you do better!
Describe the role of each of the following in predicting molecular geometries: a. unshared electron pairs, b. double bonds
Answers
In predicting molecular geometries, both unshared electron pairs and double bonds play crucial roles in determining the arrangement of atoms in a molecule.
a. Unshared electron pairs, also known as lone pairs or nonbonding electron pairs, refer to the electrons that are not involved in bonding between atoms. These electron pairs exert a significant influence on molecular geometry as they repel other electron pairs, including bonded electron pairs, creating regions of electron density around the central atom. According to VSEPR theory (Valence Shell Electron Pair Repulsion theory), the arrangement of these electron pairs and bonded electron pairs determines the shape of the molecule. The presence of unshared electron pairs affects bond angles and can lead to distortions in the idealized bond angles based on the number of bonded electron pairs. For example, in a molecule with one unshared electron pair, the bond angle might be smaller than the ideal angle due to the repulsion between the unshared pair and the bonded pairs.
b. Double bonds, on the other hand, involve the sharing of two pairs of electrons between two atoms. These double bonds influence molecular geometry by constraining the rotation and movement of atoms around them. The presence of a double bond introduces rigidity to the molecule, as the double bond prevents free rotation around the bond axis. Consequently, the atoms on either side of the double bond are fixed in position, affecting the overall shape of the molecule. For instance, a molecule with a double bond might exhibit a planar geometry or a bent geometry, depending on the number and arrangement of the other atoms or electron pairs in the molecule.
In summary, unshared electron pairs and double bonds both impact the molecular geometry of a compound. Unshared electron pairs contribute to the repulsion and affect bond angles, while double bonds introduce rigidity and limit the molecular flexibility, thereby influencing the arrangement of atoms in the molecule. Understanding the role of these factors is crucial for predicting and explaining the three-dimensional structure of molecules.
Learn more about Molecular Geometry :
https://brainly.com/question/29650255
#SPJ11
Question 4(Multiple Choice Worth 1 points)
(02.01 LC)
Who proposed that an atom is a sphere with negative electrons?
James Chadwick
OJ.J. Thomson
O Niels Bohr
O Emest Rutherford
Answers
Answer:
OJ.J. Thomson
Explanation: