Which element's atoms have a larger covalent
radius than atoms of silicon?
sodium
carbon
sulfur
chlorine
Answers
Answer:
Explanation:chlorine
Related Questions
In your OWN words explain how season are formed
Answers
Answer: Weather currents change wind tempature and there for change the weather of the seasons.
Seasons are formed through the Earth orbiting the sun and the Earth rotating.
absorbs 36.5 kJ of energy when it is vaporized.
21. Given that a substance has a molar mass of 259.0 g/mol
and a 71.8 g sample of the substance absorbs 4.307 kJ
when it melts,
a. calculate the number of moles in the sample.
b. calculate the molar enthalpy of fusion.
Answers
The number of moles in the sample of the heat change is 0.277 moles.
Heat change calculation.
a. To calculate the number of moles in the sample, we first need to calculate the number of grams in one mole of the substance:
Molar mass = 259.0 g/mol
So, one mole of the substance weighs 259.0 g.
Next, we can use the mass of the sample (71.8 g) and the molar mass to calculate the number of moles:
Number of moles = mass / molar mass
Number of moles = 71.8 g / 259.0 g/mol
Number of moles = 0.277 mol
Therefore, there are 0.277 moles in the sample.
b. To calculate the molar enthalpy of fusion, we use the formula:
q = nΔHfus
where q is the heat absorbed (in kJ), n is the number of moles, and ΔHfus is the molar enthalpy of fusion (in kJ/mol).
We are given that the sample absorbs 4.307 kJ when it melts, and we calculated in part a that the sample contains 0.277 moles. Therefore, we can plug in these values to solve for ΔHfus:
4.307 kJ = 0.277 mol x ΔHfus
ΔHfus = 4.307 kJ / 0.277 mol
ΔHfus = 15.55 kJ/mol
Therefore, the molar enthalpy of fusion of the substance is 15.55 kJ/mol.
Learn more about heat change below.
https://brainly.com/question/28912732
#SPJ1
Chemists can identify the composition of some unknown salts by conducting a flame test. When potassium salts are heated in a flame, a purple color is observed.
This is due to the movement of electrons between energy levels. What is the electron configuration of a potassium atom at ground state?
answer choices
1s2; 2s2; 2p6; 3s2; 3p6; 4d1
1s2; 2s2; 2p6; 3s2;3p6; 3d1
1s2; 2s2; 2d6; 3s2; 3d6; 4s1
1s2; 2s2; 2p6; 3s2; 3p6; 4s1
Answers
A potassium atom's ground state electron configuration is 1s2, 2s2, 2p6, 3s2, 3p6, 4s1.
What substance is electronic configuration 1s2 2s2 2p6 3s2 3p6 4s1?An atom's electron configuration is a picture of how electrons are arranged in relation to orbital shells and subshells. Consequently, this is potassium's electron configuration.
How can you express a whole electron configuration in writing?Making Electron Configurations in Writing. Write the energy level (the period) first, then the subshell that needs to be filled, and finally the superscript, which indicates how many electrons are in that subshell. The atomic number, Z, is the sum of all the electrons.
To know more about electron configuration visit:-
https://brainly.com/question/29757010
#SPJ4
The heat of fusion Δ, of benzene (C6H6) is 10.6 kJ/mol. Calculate the change in entropy AS when 2.3 g of benzene freezes at 56 °C
Answers
When benzene is cooled below its freezing point of 5.5 °C, it transforms from a liquid to a solid. The amount of energy released when a substance transforms from a liquid to a solid is referred to as the heat of fusion. The heat of fusion Δ of benzene (C6H6) is 10.6 kJ/mol.
To calculate the change in entropy AS when 2.3 g of benzene freezes at 56 °C, first, we need to calculate the number of moles of benzene that is present.Number of moles of benzene= Mass of benzene / Molar mass of benzeneMolar mass of benzene = 6 x 12 + 6 x 1 = 78 g/molMoles of benzene = 2.3 g / 78 g/mol= 0.0294871795 molHeat released = Heat of fusion x Moles of benzeneHeat released = 10.6 kJ/mol x 0.0294871795 mol= 0.312820513 kJEntropy is the measure of the randomness or disorderliness of a system. The change in entropy can be determined using the formula,ΔS = Q / TWhere Q is the heat released by the system and T is the temperature in Kelvin. The temperature in Kelvin can be obtained by adding 273.15 to the given temperature of 56 °C.T = 56 °C + 273.15= 329.15 KΔS = Q / TΔS = 0.312820513 kJ / 329.15 KΔS = 0.0009503 kJ/KThe change in entropy when 2.3 g of benzene freezes at 56 °C is 0.0009503 kJ/K.
For more information on benzene visit:
brainly.com/question/31837011
#SPJ11
Energy must be removed from a liquid to change it to a solid.
True or false?
Answers
Answer: F
Explanation:
Brainlist me
adding nh4no3 to water will produce a basic solution.
Answers
The statement adding NH₄NO₃ to water will produce a basic solution is False. Adding NH₄NO₃ (ammonium nitrate) to water will produce an acidic solution, not a basic one.
Ammonium nitrate is a salt formed by the reaction of a weak base (NH₄OH, ammonium hydroxide) and a strong acid (HNO₃, nitric acid). When dissolved in water, ammonium nitrate dissociates into ammonium ions (NH₄⁺) and nitrate ions (NO₃⁻).
The ammonium ion acts as a weak acid and can donate a proton (H⁺) to water, resulting in the formation of hydronium ions (H₃O⁺). This leads to the release of hydrogen ions, making the solution acidic. Therefore, the addition of NH₄NO₃ to water will result in an acidic solution rather than a basic one.
It's important to note that the pH of the resulting solution will depend on the concentration of NH₄NO₃ and the extent of its dissociation.
To know more about Ammonium nitrate refer here
https://brainly.com/question/5148461#
#SPJ11
Complete question :
Adding NH₄NO₃ to water will produce a basic solution. T/F
you are given the reaction cu hno3 cu(no3)2 no h2o. which element is oxidized? which element is reduced?
Answers
The reaction of copper with nitric acid takes place in two stages: at the first stage, the acid oxidizes the copper to copper oxide, releasing nitrogen dioxide
To determine which element is oxidized and which element is reduced, we need to examine the changes in oxidation states of the elements involved.
In the given reaction:
Cu + HNO₃ ⟶ Cu(NO₃)₂ + NO + H₂O,
Rhe element Cu (copper) is oxidized, while the element H (hydrogen) in HNO3 is reduced.
∴ Balanced equation is,
3Cu+8HNO₃⟶3Cu(NO₃)₂+2NO+4H₂O.
The reactions between copper and nitric acid are examples of oxidation-reduction reactions, where gaining electrons reduces one element and losing them oxidizes the other.
The reaction of copper with nitric acid takes place in two stages: at the first stage, the acid oxidizes the copper to copper oxide, releasing nitrogen dioxide; at the second stage, copper oxide reacts with new portions of acid, forming copper nitrate Cu(NO₃)₂.
To know more about electrons, visit:
https://brainly.com/question/12001116
#SPJ11
In the reaction Cu + HNO3 -> Cu(NO3)2 + NO + H2O, copper (Cu) is oxidized, going from an oxidation state of 0 to +2, and nitrogen (N) is reduced, going from an oxidation state of +5 to +2.
In the given reaction, Cu + HNO3 -> Cu(NO3)2 + NO + H2O, copper (Cu) is oxidized and nitrogen (N) is reduced.
To determine the element being oxidized, we need to identify the species that loses electrons. In this case, copper (Cu) goes from an oxidation state of 0 in Cu to an oxidation state of +2 in Cu(NO3)2. This indicates that copper loses two electrons during the reaction, making it the element that is oxidized.
On the other hand, to determine the element being reduced, we need to identify the species that gains electrons. In this reaction, nitrogen (N) goes from an oxidation state of +5 in HNO3 to an oxidation state of +2 in NO. This indicates that nitrogen gains three electrons during the reaction, making it the element that is reduced.
In summary, in the reaction Cu + HNO3 -> Cu(NO3)2 + NO + H2O, copper (Cu) is oxidized and nitrogen (N) is reduced.
Learn more about oxidation
https://brainly.com/question/25886015
#SPJ11
How many fluorine atoms are present in 6.30 g of C2F4
Answers
Fluorine atoms = 1.517 x 10²³
Further explanationThe mole is the number of particles contained in a substance
1 mol = 6.02.10²³
Moles can also be determined from the amount of substance mass and its molar mass
\(\large{\boxed{\boxed{\bold{mol=\frac{mass}{molar\:mass}}}}\)
Proust stated the Comparative Law that compounds are formed from elements with the same Mass Comparison so that the compound has a fixed composition of elements
Mass of F\(\tt mass~F=\dfrac{4.Ar~F}{MW~C_2F_4}\times mass~C_2F_4\\\\mass~F=\dfrac{4.19}{100}\times 6.3=4.788~gr\)
mol of F\(\tt =\dfrac{4.788}{19}=0.252\)
number of Fluorine atoms\(\tt 0.252\times 6.02\times 10^{23}=1.517\times 10^{23}\)
Mark leamed that the boiling points are indicative of the relative strength of the secondary forces. Which of the following substances would you oxpect to have the highest boling point? NH 3
CH 4CO2
H2
CO
All of them have the same boling point
Answers
The substance which has the highest boiling point is \(NH_3\).
Ammonia is expected to have the highest boiling point among the given options. This is due to the fact that ammonia molecules are polar and capable of hydrogen bonding between molecules. Hydrogen bonding is a strong intermolecular force that requires a significant amount of energy to break, resulting in a higher boiling point.
In contrast, methane and carbon dioxide are nonpolar molecules and have only weak van der Waals forces, resulting in relatively low boiling points. hydrogen and CO (carbon monoxide) are also nonpolar molecules with only weak van der Waals forces, resulting in even lower boiling points than \(CH_4\) and \(CO_2\).
Thus, ammonia would have a higher boiling point than the other molecules listed due to its polar nature and the presence of hydrogen bonding.
To learn about boiling point:
https://brainly.com/question/30039297
#SPJ4
Which factor causes a decrease in the rate of dissolution?
Answers
There are several factors that can cause a decrease in the rate of dissolution:
Decrease in temperature: As the temperature decreases, the kinetic energy of the particles decreases, and the rate of dissolution also decreases.
Increase in solute concentration: If the solution is already saturated with solute, then adding more solute will cause it to become supersaturated, which can cause a decrease in the rate of dissolution.
Increase in pressure: Increasing the pressure can force more solute into the solution, but it can also cause an increase in the solubility of the solute, which can cause a decrease in the rate of dissolution.
Decrease in surface area: If the solute is in the form of large particles, then breaking it down into smaller particles will increase the surface area available for dissolution and increase the rate of dissolution. Conversely, decreasing the surface area will decrease the rate of dissolution.
Formation of a precipitate: If the solute is capable of forming a precipitate in the solution, then the rate of dissolution may decrease as the solute is removed from the solution and deposited as a solid.
~~~Harsha~~~
12)
In the classification of matter chart, letter B. would be a(n)
A) acid.
B) compound.
C) element.
D) solution.
Answers
6a) What would the DIGITS (with the decimal) of 0039283 be in scientific notation?
Answers
Answer:
3.9283 x 10^-3
Explanation:
Assuming there is a missing decimal in front of your first zero
what is the balanced equation of Ag2S---->_Ag+_S8
Answers
i hope this helps a little
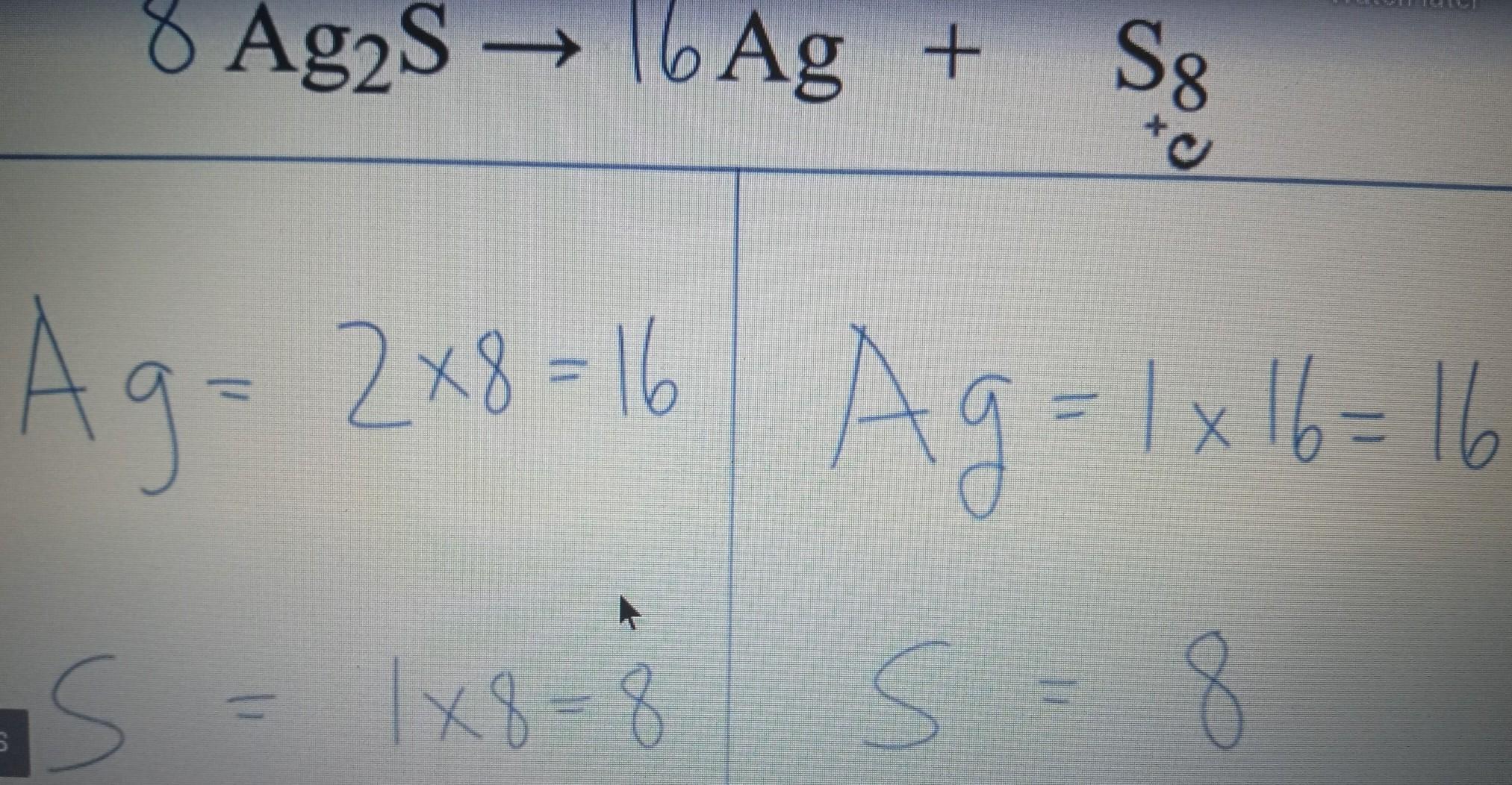
An element A has an atomic number of 11 and another element B has an atomic number 17 (A
and B are not actual chemical symbols of elements)
i. What group on the periodic table does A belong?
ii. What type of bond will be formed between A and B? Explain.
(2mks
(1ml
iii.
Write the formula of the compound that will be formed between A and B.
(lm
b. Arrange the species X, X5+ and X. in order of increasing size.
Answers
Answer:
can anyone tell me how to get +7!67:$'!$
PLZZZZZZ HEEEELLLLPPPPP!!!!!!!!!!!!!!
What is the relationship between the length of a lever and the force required? (where should the fulcrum be to get the best advantage?)
Answers
Answer:
The answer should be fulcrum
Explanation:
It would be easier if you game me choices to pick from
Question 3 (5 points) (02.06 MC) The table compares some characteristics of two atoms_ Charged Particles Atom Number 0f Neutrons Mass Number] Use the table t0 determine the number of protons for each atom: Then_ choose the statement below that Is true about the two atoms_ points) Atom X and Atom are in the same row; but not the same family; on the periodic table Atoms X and Atom Y are in the same family; but not the same row; in the periodic table; Atom Xis in a column to the right of Atom Y in the periodic table: Atom X and Atom occupy the same position in the periodic table because they are isotopes'
Answers
Atoms are basically the smallest units of matter. They are made up of protons, neutrons and electrons.
What is the Periodic table?The periodic table is a tabular arrangement of the chemical elements which are organized on the basis of their atomic numbers, electron configurations, and recurring chemical properties. The periodic table lists the elements in order of increasing atomic number and grouped into rows (periods) and columns (groups). The elements in the same column have similar chemical properties, with the elements in the same row having similar outer electron configurations.
Atom X: Number of protons = 6
Atom Y: Number of protons = 6
b. Atoms X and Atom Y are in the same family; but not the same row; in the periodic table.
Hence, Option B is correct.
To know more about periodic table, visit:
brainly.com/question/15987580
#SPJ4
The question is:
Question 3 (5 points) (02.06 MC) The table compares some characteristics of two atoms_ Charged Particles Atom Number 0f Neutrons Mass Number] Use the table t0 determine the number of protons for each atom: Then_ choose the statement below that Is true about the two atoms_ points) Atom X and Atom are in the same row; but not the same family; on the periodic table Atoms X and Atom Y are in the same family; but not the same row; in the periodic table; Atom Xis in a column to the right of Atom Y in the periodic table: Atom X and Atom occupy the same position in the periodic table because they are isotopes.
How do American cities now protect their water resources
Answers
Answer:
They can use man made materials or they can also have designed areas that people can’t go into
Explanation:
what is the ph of a solution that has a [h3o ] = 4.5 x 10-5 ?
Answers
The pH of a solution that has a [H3O+] concentration of 4.5 × 10⁻⁵ is 4.35.
Given that
[H₃O⁺] = 4.5 × 10⁻⁵
We know that
pH = -log[H₃O⁺]pH = -log(4.5 × 10⁻⁵)
pH = -log 4.5 - log 10⁻⁵
pH = -log 4.5 + 5pH = 4.35
Hence, the pH of a solution that has a [H₃O+] concentration of 4.5 × 10⁻⁵ is 4.35.
Therefore, the pH of the given solution is 4.35.
To know more about pH, visit:
https://brainly.com/question/2288405
#SPJ11
Use the drop down menus to answer the questions.
In what type of plate boundary did subduction occur?
In what type of plate boundary did new crust form?
In what type of plate boundary did mountains form?
In what type of plate boundary did volcanoes form?
In what type of plate boundary did a river change its path?
Answers
Convergent boundary type of plate boundary occurred at the subduction.
Divergent boundary type of plate boundary occur at the subduction.
Convergent boundary type of plate boundary occur at new crust form.
Convergent boundary type of plate boundary that volcanoes form.,
Transform boundary type of plate boundary occurs at a river.What are different types of boundaries in details?
What are the boundaries in details?An Earth region known as a convergent border occurs where two or more lithospheric plates collide. Subduction is the inevitable sliding of one plate beneath the other. The Wadati-Benioff zone, a plane with a high frequency of earthquakes, can be used to designate the subduction zone.
The Pacific Plate slides past the North American Plate as it moves northwest, establishing the transform plate border, which is a vast zone. In addition to the San Andreas Fault, it has other smaller faults. 11
When two tectonic plates diverge, a diverging border is created. As magma (molten rock) rises from the Earth's mantle to the surface and solidifies to form new oceanic crust, earthquakes frequently occur along these borders. Divergent plate boundaries can be seen, for instance, in the Mid-Atlantic Ridge.
Learn more about plate boundary at:
https://brainly.com/question/14565919
#SPJ1
PLEASE HELP DUE TODAY AGAIN FOR SCIENCE !!!!!
What do the most abundant elements in Earth’s atmosphere have in common?
Answers
Nitrogen is an element that is present in the atmosphere but in a large amount and also makes up the earth's atmosphere up to 78%.
Which element is present in a large amounts in the atmosphere?We know that the most abundant elements which are present in the earth's atmosphere are gases, so gas is the most common and abundant element in the earth's atmosphere.
The most abundant naturally occurring gas in the earth's atmosphere is nitrogen (N2). Nitrogen makes up 78% of the earth's atmosphere while oxygen is the second most abundant naturally occurring gas in the earth's atmosphere which is about 21%. The inert gas Argon is also present in the earth's atmosphere and is the third most abundant gas in the earth's atmosphere which is about 0.93%.
So we can conclude that the most abundant element in the earth's atmosphere is nitrogen which makes the atmosphere up to 78%.
Learn more about Earth's Atmosphere here: https://brainly.com/question/1844421
#SPJ1
What is the answer?

Answers
Answer:
Option A
Explanation:
The atomic number is the number of protons in the nucleus of an atom.
How many moles of fluorine gas are in 54.1 grams of fluorine gas?
Answers
Answer: 2.85 moles of flourine
Explanation:
find the atomic/molar mass of flourine on your periodic table.
multiply the given mass (54.1g) by (1 mole of flourine/atomic mass of flourine)
54.1g flourine * 1mole of flourine/18.998g flourine
(g flourine) in the above equation cancel each other.
54.1/18.998 = 2.85 mole of flourine
Which of the following comes first in the name of an ionic compound?(1 point)
the positively charged ion
the positively charged ion
the neutral ion
the neutral ion
the negatively charged ion
the negatively charged ion
the number of ions present
Answers
Answer:
Positively charged ion
Explanation:
Customary nomenclature rules
In naming an ionic compound, the name of the the positively charged ion comes first.
An ionic compound is a compound formed when a positively charged ion and a negatively charged ion come together. Ionic substances are held together by electrostatic attraction.
When naming an ionic compound, it is normal to name the the positively charged ion first, followed by the name of the negatively charged ion.
Learn more: https://brainly.com/question/19293051
mrs. smith's class is doing an experiment with thermometers. they fill up a cup with boiling water and set it on the counter. they plan to check the temperature of the water in the cup after 5, 10, 15, and 30 minutes. what will mrs. smith's class observe about the temperature of the water after 30 minutes? responses a the temperature will increase first and then decrease.the temperature will increase first and then decrease. b the temperature of the water will decrease.the temperature of the water will decrease. c the temperature of the water will increase greatly.the temperature of the water will increase greatly. d the temperature of the water will stay the same.
Answers
The temperature of water after 30 minutes will decrease. The correct option to this question is B.
The thermometer may blow up if the temperature exceeds the permitted limit. Since boiling water is known to have a temperature in the range of 100 C, a standard clinical thermometer cannot be used to measure its temperature. Query: Laboratory Boiling water's temperature can be determined using thermometers.
A thermometer's operation is an illustration of how a liquid can be heated and cooled. The molecules of the liquid in the thermometer move more quickly when it is heated, which causes them to move somewhat farther apart. The thermometer moves upward as a result.
For more information on temperature kindly visit to
https://brainly.com/question/11464844
#SPJ4
Which statement is always true about a reversible chemical reaction?
A. The concentration of reactants is higher than that of the products.
B. The products can form reactants, and the reactants can form products.
C. The concentrations of reactants and products are not constant.
D. The concentration of the products is higher than that of the reactants.
Answers
What does light travel in?
Answers
Answer:
Light travels as a wave.
Explanation:
But unlike sound waves or water waves, it does not need any matter or material to carry its energy along. This means that light can travel through a vacuum—a completely airless space. (Sound, on the other hand, must travel through a solid, a liquid, or a gas.)
16.2296 rounded in significant figure
Answers
16.2296 has 6 significant figures and 4 decimals. 16.2296 rounded to 5 sig figs is 16.230, to 4 sig figs is 16.23, and to 3 sig figs is 16.2. To count the number of sig figs in 16.2296, count all 6 digits since it has no insignificant digits (all digits are significant).
Result 16.2296
Result 16.2296Sig Figs 6 (16.2296)
Result 16.2296Sig Figs 6 (16.2296)Decimals 4 (16.2296)
Result 16.2296Sig Figs 6 (16.2296)Decimals 4 (16.2296)Scientific Notation 1.62296 × 101
E-Notation. 1 .62296e+1
.62296e+1Words sixteen point two two nine six
make me brainalist and keep smiling dude
The ionization energy trend on the periodic table is highest to the (left/right) and upper corner
______________
Answers
Answer: right
Explanation:
how do the hydrogen bonds between water molecules compare to the covalent bonds within water molecules?
Answers
Covalent bonds within water molecules are stronger than hydrogen bonds between them.
What is covalent bond?A covalent bond is a sort of chemical link created when two or more atoms share electrons. In a covalent bond, the atoms share electrons in order to complete their valence shells, creating a stable and powerful link.
In water molecules, there are two basic kinds of chemical bonding: covalent bonds and hydrogen bonds. Between the hydrogen atoms of one water molecule and the oxygen atom of another water molecule, hydrogen bonds are created. Compared to covalent bonds, they are relatively weak interactions, yet they are potent enough to have a significant impact on how water behaves.
The oxygen and hydrogen atoms of a single water molecule, on the other hand, create covalent connections. Since covalent bonds require the exchanging of electrons between atoms, they are stronger than hydrogen bonds. These connections form a solid and cohesive structure that gives water its special characteristics.
To know more about covalent bond, visit:
https://brainly.com/question/19382448
#SPJ4
chemistry hw due in 2 hours... HELP!!!

Answers
Answer:
36kcal
Explanation:
There are three things that occur in the process not converting ice to steam. They are;
I) fusion of ice
ii) heating of water to 100°C
iii) vaporization of water at 100°C to yield steam
Hence, total heat required to convert ice to steam=
Latent heat of fusion of ice + heat required to raise the temperature of water to 100°C + Latent heat of vaporization of water
Thus;
H= mLfus + mcθ + mLvap
H= m(Lfus + cθ + Lvap)
H= 50(80 + 1(100) + 540)
H= 36000cal
H= 36kcal