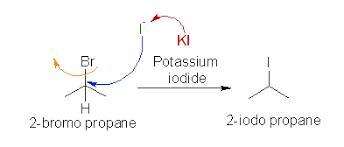
Write equations for the following: (a) a substitution reactionbetween 2-bromopropane and KI;
Answers
The required equation for the substitution reaction between 2-bromopropane and KI is given by ,
CH3-CH(Br)-CH3 + KI ( Potassium iodide )==> CH3-CH(I)-CH3
What is substitution reaction ?
it is type of chemical reaction in which the the functional group of one chemical compound is replaced by the another functional group .
the types of substitution reactions are electrophilic substitution reaction( aromatic and aliphatic substitution reaction) and nucleophilic substitution reaction (SN1 and SN2 nucleophilic reaction ) .
Learn more substitution reaction here :
brainly.com/question/10143438
#SPJ4
Related Questions
Madison Anybody graduated from college 5.5 months ago. It took 5 months to find a job, but now she’s gainfully employed in a career aligned to her major, and it’s your job to help her manage her debt in the most responsible way possible.
Answers
Here are three steps Madison can take to molten manage her debt in the most responsible way possible:1. Create a budget: The first thing Madison should do is create a budget.
Madison should start by listing all of her monthly expenses, including rent, utilities, groceries, transportation, and any debt payments. She should also include her monthly income.2. Prioritize her debt: Madison should prioritize her debt by paying off the debt with the highest interest rate first. This will help her save money in the long run by reducing the amount of interest she has to pay. Madison should also consider consolidating her debt to simplify her payments and potentially lower her interest rates.
Madison should prioritize her debt by paying off the debt with the highest interest rate first. This will help her save money in the long run by reducing the amount of interest she has to pay. Madison should also consider consolidating her debt to simplify her payments and potentially lower her interest rates.3. Build an emergency fund: Finally, Madison should build an emergency fund. This will help her avoid relying on credit cards or other forms of debt in case of unexpected expenses, such as a car repair or medical bill. Madison should aim to save at least three to six months’ worth of expenses in her emergency fund.
To know more about molten visit:
https://brainly.com/question/30404513
#SPJ11
The table below contains characteristics of four different elements. Characteristics of Four Elements Element Number of Protons Number of Valence Electrons 1 ca 4 2. 11 1 1 3 18 8 4 37 1 1 What conclusion can be made from the information above? Element 3 is a metal and Element 1 is a metalloid. Element 2 is the same element as Element 3. Element 1 has very similar chemical properties as Element 4. Element 2 has very similar chemical properties as Element 4.

Answers
Answer:
The correct option is "Element 2 has very similar chemical properties as Element 4"
Explanation:
Both element 2 and element 4 have the same number of valence electrons; meaning they will be found in the same group (group 1) of the periodic table. Elements in the same group of the periodic table exhibit similar chemical properties. For confirmation, element 2 is sodium (based on the atomic number of 11 provided) while element 4 is rubidium (based on the atomic number of 37 provided) - the two elements are found in group 1 of the periodic table.
NOTE: Number of protons is the same as atomic number
Which substance has AHf defined as O kJ/mol?
A. H20 (s)
B. Ne (I)
C. F2 (g)
D. CO2 (g)
It’s C.
Answers
Answer:
(C). is the answer, I think
Fluorine in gaseous form is a substance which has atoms far away from one another has ΔHf as 0 kj/mole.
What is an atom?An atom is defined as the smallest unit of matter which forms an element. Every form of matter whether it is solid,liquid , gas is made up of atoms . Each atom has a nucleus which is composed of protons and neutrons and shells in which the electrons revolve.
The protons are positively charged and neutrons are neutral particles and hence the nucleus is positively charged. The electrons which revolve around the nucleus are negatively charged and hence the atom as a whole is neutral and stable due to presence of oppositely charged particles.
Atoms of the same element are similar as they have number of sub- atomic particles which on combination do not alter the chemical properties of the substances.
Learn more about atom,here:
https://brainly.com/question/29695801
#SPJ7
When producing a soluble salt in a reaction between an acid and an alkali, how can you prepare dry solid crystals from the solutions
1)evaporation/crystallization
2)filtration
3)condensation
4)nuetralisation
Answers
Explanation:
Making a salt from an alkali
If you are using an alkali - which is a soluble base - then you need to add just enough acid to make a neutral solution (check a small sample with universal indicator paper).
Warm the salt solution to evaporate the water. You get larger crystals if you evaporate the water slowly.
calculate [oh−] for 1.1×10−3 m sr(oh)2.
Answers
The hydroxide ion concentration [OH-] in a 1.1×10−3 M solution of Sr(OH)2 can be calculated using the solubility product constant (Ksp) for the compound. The final concentration of [OH-] in the solution is 2.4×10−4 M.
1. The Ksp for Sr(OH)2 is 5.4×10−12, which represents the equilibrium constant for the dissolution of Sr(OH)2 in water. By assuming that the dissociation of Sr(OH)2 in water is complete, we can calculate the molar concentration of [OH-] from the stoichiometry of the reaction.
2. The solubility product constant (Ksp) is the equilibrium constant for the dissolution of a sparingly soluble salt in water. It represents the concentration of the ions produced when the solid salt dissolves. For Sr(OH)2, the Ksp is given as: Sr(OH)2 ⇌ Sr2+ + 2OH−
Ksp = [Sr2+][OH−]2 = 5.4×10−12
3. The stoichiometry of the reaction shows that for every one mole of Sr(OH)2 that dissolves, it produces one mole of Sr2+ ions and two moles of OH− ions. Therefore, if we assume that all of the Sr(OH)2 has dissociated completely, then the molar concentration of [OH−] is twice that of [Sr(OH)2]. [OH−] = 2[ Sr(OH)2]
[OH−] = 2 × 1.1×10−3 M
[OH−] = 2.2×10−3 M
4. However, we need to take into account the fact that [Sr2+] and [OH−] will recombine to form Sr(OH)2, which will affect the concentration of [OH−]. To calculate the concentration of [OH−] at equilibrium, we can use the quadratic equation to solve for x in the expression for the Ksp:
Ksp = [Sr2+][OH−]2 = (x)(2x)2 = 5.4×10−12
x = [OH−] = 2.4×10−4 M
5. Thus, the final concentration of [OH−] in the solution is 2.4×10−4 M, which is much smaller than the initial concentration of 2.2×10−3 M. This indicates that the reaction has reached equilibrium, with most of the Sr2+ and OH− ions combining to form solid Sr(OH)2.
Learn more about equilibrium constant here: brainly.com/question/29809185
#SPJ11
please answer truthfully:))

Answers
Answer:
Fast, Direction, Time
Explanation:
how can you find the michaelis constant, km, from a saturation plot?
a. It is equal to the Vmax
b. It is the subtrate concentration at Vmax
c. It is the subtrate concentration at ½ Vmax
d. It is the subtrate concentration at ¼ Vmax
e. It is the reaction rate at ½ subtrate concentration
Answers
The Michaelis constant (Km) (C) is the substrate concentration at ½ Vmax.
Enzyme kinetics refers to the study of the chemical reactions and mechanisms of enzymes. This branch of biochemistry focuses on the analysis of how enzymes work and how they can be controlled. In general, the Michaelis-Menten equation is used to explain the kinetics of enzyme-catalyzed reactions. It demonstrates the relationship between the reaction rate, substrate concentration, and enzyme activity.
Therefore, in enzyme kinetics, Km is the Michaelis constant, and it reflects the affinity of an enzyme for its substrate. It is defined as the substrate concentration that corresponds to half of the maximum velocity (Vmax) of the reaction. In other words, at Km, the reaction rate is half of the Vmax.
Therefore, the Michaelis constant (Km) can be determined from a saturation plot by identifying the substrate concentration at half of the maximum velocity (Vmax/2).
Know more about Enzyme kinetics here :
https://brainly.com/question/13635701
#SPJ11
Petroleum is a fossil fuel containing many different carbon compounds. If the carbon atoms in petroleum have been in the ground for 100 million years, what fraction of the initial 14C atoms is still there
Answers
Thus, Option (C) Glucose has a maximum of 72 C-atoms per gram. The components of fossil fuels are decaying plants and animals.
These fuels may be burned to provide energy and can be found in the crust of the Earth. An example of a fossil fuel is coal, as well as oil and natural gas. Due to the hydrocarbons and other carbon-based compounds they contain, fossil fuels are valuable as energy sources. Any of a class of organic molecules made solely of carbon and hydrogen are known as hydrocarbons. The hydrogen atoms adhere to the carbon atoms, which serve as the "skeleton" or framework. Because they were generated from the petrified, buried remains of plants and animals that once lived millions of years ago, coal, crude oil, and natural gas are all referred to as fossil fuels.
The complete question is- Petroleum is a fossil fuel containing many different carbon compounds. If the carbon atoms in petroleum have been in the ground for 875 million years, what fraction of the initial 14C atoms is still there?
Learn more about carbon here-
https://brainly.com/question/22530423
#SPJ4
Determine the molecular formula for a compound that has a Mass of 392.2 grams and consists of 0.70g of chromium,0.65g of sulfur and 1.30 grams of oxygen
Answers
Explanation:
yuyfjkvg8hvcrnkffhbbbvvggh
what’s the answer ?????

Answers
option A is the correct one
It represents Mp orBp of a substance at a specific pressure
c+o2=co
Al+s=al2s3
Al(oH)3+H2So4=al2(So4)2+H20
BALANCE THE REACTION
TYSM
Answers
1)
2C + O² => 2CO
2)
2Al + 3S => Al²S³
3)
2Al(OH)³ + 3H²SO⁴ => Al²(SO4)³ + 6H²O
A tin box has a volume of 60.0 cubic centimeters and a mass of 465 grams. Calculate its density.
Answers
Answer:
hi!!
Explanation:
first we search for the volumic mass :
volumic mass = mass/volum=465/60.0 = 7.75 gper cubic centimeters
density = volumic mass of the tin box / volumic mass of water = 7.75/ 1 (g per cubic centimeters)= 7.75
enter your answer in the provided box. how many milliliters of 1.16 m naoh must be added to 175 ml of 0.20 m nah2po4 to make a buffer solution with a ph of 7.30? ml
Answers
The volume of the Naoh that is required was =4.074
What is the use of the buffer solution ?
A buffer is an aqueous solution made up of a weak acid and its salt (acid buffer) or a weak base and its salt (base buffer) (basic buffer). When a small amount of strong acid or base is added to it, its pH changes very little, and it is thus used to prevent the pH of a solution from changing.
Buffer solutions are utilised in several chemical applications. Blood is one example of a natural buffer solution. The natural pH of human blood is 7.4. Many people suffer from severe anxiety and alkalosis. Alkalosis is a condition in which the blood pH is abnormally high. The opposite situation is known as acidosis, which occurs when the pH of the blood exceeds 7.4.
naoh+nah2po4 ------------> h2o+na3po4
pka=3.39
7.30=3.39+(log(h2o/naoh)
log(h2o/naoh)=7.30-3.39
=3.91
=10^log(h2o/naoh)=10^3.91
=4.074
The volume required was=4.074
To learn more about buffer solution follow the given link: https://brainly.com/question/27371101
#SPJ4
How many moles are in 20 grams of O₂ gas?
Answers
Balancing Equations.

Answers
Answer:
4Al +3O2 --> 2Al2O3
Explanation:
they are balanced
For to complete valancy oxygen needs two electron.
Now the balancing can be performed as;
4Al + 3O2———— 2Al2O3
EXPLANATION:
Aluminium have 3 valance electrons. So 4 aluminium have 12 electrons. Each oxygen can take 2 electrons so 6 Oxygen atoms can take 12 electrons.
So the number of loss and gain of electrons are equal and the number of elements on the reactant side is equal to the product side. Therefore, the equation is said to be a balance.
Why is resistance useful in
lightbulbs?
A. It allows the filament to cool off and get dim.
B. It allows the filament to heat up and glow.
C. It allows the filament to heat up and get dim.
D. It allows the filament to cool off and glow.
Answers
Which statement BEST describes the difference between groups and periods in the periodic table of elements?
A) Elements in periods have similar properties, while elements in groups have gradually changing properties
B) Elements in periods have gradually changing chemical properties, while elements in groups have similar properties.
C) Elements in periods are found in vertical columns, while elements in groups are found in horizontal rows.
D) Elements in periods are mostly metals, while elements in groups are mostly metalloids.
Answers
Although elements in grouping have similar properties, those in period have gradually shifting chemical properties.
Describe element.Any compound that cannot be broken down into simpler chemicals by regular chemical processes is referred to as a chemical element or element. The building blocks from which every matter is made are called elements. a molecule that cannot be chemically divided into simpler substances.
What are the four primary chemical elements?About 96% of a human body is made up of just just four substances: carbon (C), oxygenation (O), hydrogen (H), and nitrogen (N). There are 25 identified elements that are necessary for life. According to the required quantity, this graphic divides the important components into three major groupings.
To know more about elements visit:
https://brainly.com/question/24407115
#SPJ1
Answer:
It's B
Explanation:
Trust me, I have 50 million power
List the four common units of pressure and their relationship to 1 atmosphere (atm).
Answers
Answer:
Pascal (1 N/m²) (Pa) 101,325 Pa = 1 atm.
Pounds per square inch (psi) 14.7 psi = 1 atm.
Torr (1 mmHg) 760 torr = 1 atm.
Inches of Mercury (in Hg) 29.92 in Hg = 1 atm.
Atmosphere (atm) 1 atm = 1 atm.
Hope this helps :)
Pls brainliest...
CAN SOMEONE PLEASE HELP ME OUT WITH THESE 2 QUESTIONS? ILL GIVE YOU A GOOD RATING!!

Answers
MULTIPLE CHOICE QUESTION
Gain or Lose
Magnesium (Mg) has 2 valence electrons. Will
the Mg atom gain or lose electrons to form an
ion?
• Elements > 4 valance e-
gain e-
• Elements < 4 valance e-
lose e-
Neither
Lose
Gain
Answers
The dog has a mass of 57kg and the boy has a mass of 48 kg. Who has more kinetic energy?

Answers
Answer:
The Dog
Explanation:
The more mass something has the more kinetic energy it has in it.
Are the following chemical equations reversible or irreversible?
2H2O ←→ H3O+ + OH-
HA + H2O ←→ A- + H3O+
HA + H2O → A- + H3O+
MOH → M+ + OH-
Answers
The first two chemical equations are reversible while the other two are irreversible.
What are chemical equations?Chemical equation is a symbolic representation of a chemical reaction which is written in the form of symbols and chemical formulas.The reactants are present on the left hand side while the products are present on the right hand side.
A plus sign is present between reactants and products if they are more than one in any case and an arrow is present pointing towards the product side which indicates the direction of the reaction .There are coefficients present next to the chemical symbols and formulas .
The first chemical equation was put forth by Jean Beguin in 1615.By making use of chemical equations the direction of reaction ,state of reactants and products can be stated. In the chemical equations even the temperature to be maintained and catalyst can be mentioned.
Learn more about chemical equations,here:
https://brainly.com/question/19626681
#SPJ1
When heated, the alcohol inside a thermometer
expands
O contracts
Answers
Answer:
it expands
Explanation:
this is because when it get warmer it runs out of room and makes it got up the tube.
Do you think it’s important that forensics is categorized as a science? Why or why not?
Answers
How many dimes are there in $6.00
Answers
Answer:
there are 60 dimes in 6 dollars
Which statement is incorrect about the setup of voltaic cell? a.A voltaic cell is an electrochemical cell that uses spontaneous redox reactions to generate electricity. It consists of two separate half-cells. A salt bridge also connects to the half cells. b.Salt bridge is a tube usually filled with an electrolyte solution such as KNO3(s) or KCI(s) c.The salt bridge allows a flow of ions that neutralizes the charge build up in the solution. d.In Voltaic cells, oxidation occurs at cathode and reduction occurs at anode.
Answers
The incorrect statement about the setup of a voltaic cell is (d) "In voltaic cells, oxidation occurs at the cathode and reduction occurs at the anode."
In a voltaic cell, oxidation actually occurs at the anode and reduction occurs at the cathode. This is because electrons flow from the anode (where oxidation takes place) to the cathode (where reduction takes place). The anode is the electrode where oxidation reactions take place and electrons are released, while the cathode is the electrode where reduction reactions occur and electrons are gained. To explain further, in a voltaic cell, the anode is the electrode where the oxidation half-reaction occurs. Oxidation involves the loss of electrons and the anode serves as the source of electrons. These electrons then flow through an external circuit to the cathode. At the cathode, reduction takes place, which involves the gain of electrons. The cathode acts as the site where reduction half-reactions occur, consuming the electrons that flow from the anode. Therefore, the correct statement should be: "In voltaic cells, oxidation occurs at the anode and reduction occurs at the cathode."
learn more about voltaic cell here: brainly.com/question/31729529
#SPJ11
What main type of forces must be overcome between br2 when liquid br2 dissolves into ethanol?
Answers
Dipole-induced dipole forces must be overcome between br2 when liquid br2 dissolves into ethanol.
In contrast to C2H5OH, which is a very polar molecule, bromine is non-polar and has zero dipole moment. Charges are separated within polar molecules.
When a nonpolar Br2 molecule interacts with a polar C2H5OH molecule, one half of the bromine molecule acquires a charge and the other half acquires an opposing charge. A dipole is created when two adjacent bromine molecules have different charges. Bromine molecules must thus overcome the intermolecular forces—also known as dipole-induced dipole forces—that form in order to dissolve in ethanol.
Find more on bromine at : brainly.com/question/865727
#SPJ4
small cations are attracted to colloid surfaces more strongly than large cations.
Answers
Colloids are particles that measure between 1 and 1000 nanometers in size and scatter light. They are relatively stable particles that remain suspended in a solvent and do not settle over time.
Because of their small size, colloids have a large surface area, which is a key factor in their reactivity with ions, including cations.The attraction of ions towards a colloid surface is based on their size, charge, and concentration. The charge of a cation plays a vital role in the interaction of a colloid's surface. This is because a colloid's surface carries an opposite charge to that of the cation.
Small cations have a higher charge density than larger ones, which means they are attracted more strongly to colloid surfaces.The high charge density of small cations can interact with the colloid's surface at a closer range than large cations. Because of their size, large cations cannot come as close to the colloid's surface as small cations. Hence, small cations are attracted to colloid surfaces more strongly than large cations.As a result, smaller cations are more attracted to the surface of the colloids than larger ones. This suggests that the stability of colloidal systems is affected by the size of the cation involved.
To know more about Colloids visit;-
https://brainly.com/question/30560048
#SPJ11
Match each scientist to their discovery regarding the atom
Thomson
Electrons have a charge of -1.
Rutherford
Atoms are indivisible
Millikan
Atoms have a positive nucleus
Dalton
Atoms contain electrons.
Answers
Answer:
Thomson--atoms cotain electron
Ernest Rutherford--atoms have a positive nucleus
R.A Millikan--electrons have Q=-1
Dalton--atoms are indivisible
Millikan---> Electrons have a charge of -1
Rutherford ---> Atoms have a positive nucleus
Thomson -----> Atoms contain electrons
Dalton --------> Atoms are indivisible
The atomic theory went through several modifications and different scientists proposed various models of the atom until our present conception of the atom was developed.
The atom was first defined as the smallest indivisible particle of a substance. This idea of "indivisibility" of the atom stems from Dalton's theory.
The fact that atoms were composed of negatively charged electrons was proven by the experiments of J.J Thompson using the cathode ray tube. Millikan's charge to mass experiment showed that the electron has a charge of -1.
Rutherford, in his famous gold foil experiment showed that atoms were composed of a positively charged nucleus.
Learn more: https://brainly.com/question/1596638

For the equilibrium system described by this
equation, what will happen if SO3 is removed?
The equilibrium shifts to the
Answers
Overall, if \(SO_3\) is removed from the system, the reaction rate will decrease and the equilibrium position of the system will shift towards higher concentrations of \(SO_2\) and Oxygen and lower concentrations of \(SO_3\).
The equation describes the rate of reaction for a chemical reaction involving sulfur trioxide ( \(SO_3\)) and hydrogen peroxide. If \(SO_3\) is removed from the system, the reaction rate will decrease.
The equation can be written as:
2 \(SO_3\) -> 2 \(SO_2\) + Oxygen
In this reaction, \(SO_3\) is the reactant and \(SO_2\) and Oxygen are the products. The reaction rate is determined by the rate at which the reactant is consumed. If \(SO_3\) is removed from the system, there will be fewer reactants available to participate in the reaction, which will result in a slower reaction rate.
It's important to note that if the reaction rate decreases, the equilibrium position of the system will also change. At equilibrium, the forward and reverse reactions occur at the same rate, so if the reaction rate decreases, the reaction will shift towards the products. The concentration of \(SO_2\) and Oxygen in the system will increase, and the concentration of \(SO_3\) will decrease as the reaction reaches equilibrium.
Learn more about equilibrium visit: brainly.com/question/23121462
#SPJ4
Correct Question:
For the equilibrium system described by this equation, what will happen if SO3 is removed?
Answer:
it shifts to the right
the second question is left
the third question is left
the fourth question is right
Explanation: