If four molecules of ribulose bisphosphate (RuBP) were added to a solution containing rubisco and CO2, what would be the products of the reaction
Answers
The products of the reaction that takes place between four molecules of ribulose bisphosphate (RuBP) and CO2 in a solution containing rubisco are eight molecules of 3-phosphoglycerate (3-PGA).
Rubisco is an enzyme that facilitates the binding of carbon dioxide with ribulose bisphosphate (RuBP), the primary acceptor of CO2 during photosynthesis.
The reaction that takes place between RuBP and CO2 is a critical step in the Calvin cycle.
The products of the reaction when four molecules of RuBP are added to a solution containing rubisco and CO2 are 8 molecules of 3-phosphoglycerate (3-PGA).
During the light-independent reactions of photosynthesis, the Calvin cycle begins with the enzyme rubisco catalyzing the addition of CO2 to RuBP, which generates an unstable intermediate that quickly forms two molecules of 3-PGA.
Since two molecules of 3-PGA are produced for each CO2 molecule that enters the cycle, if four RuBP molecules are present, the reaction would yield eight molecules of 3-PGA.
In conclusion, the products of the reaction that takes place between four molecules of ribulose bisphosphate (RuBP) and CO2 in a solution containing rubisco are eight molecules of 3-phosphoglycerate (3-PGA).
Learn more about Rubisco at: https://brainly.com/question/30053157
#SPJ11
Related Questions
Please a little help in this I will really appreciate it

Answers
Answer:
b is your answer...........
Kads, inc. has spent $370,000 on research to develop a new computer game. the firm is planning to spend $170,000 on a machine to produce the new game. shipping and installation costs of the machine will be capitalized and depreciated; they total $47,000. the machine has an expected life of three years, a $72,000 estimated resale value, and falls under the macrs seven-year class life. revenue from the new game is expected to be $570,000 per year, with costs of $220,000 per year. the firm has a tax rate of 21 percent, an opportunity cost of capital of 11 percent, and it expects net working capital to increase by $85,000 at the beginning of the project. what will the cash flows for this project be? (negative amounts should be indicated by a minus sign. round your answers to 2 decimal places.)
Answers
The cash flow for the given project will be for three years with Salvage value: $72,000.
To determine the cash flows for the project, we need to consider the initial investment, annual revenues and costs, depreciation, taxes, and changes in net working capital. Let's calculate each component:
Initial Investment:
Research expenses: -$370,000
Machine cost: -$170,000
Shipping and installation costs: -$47,000
Net working capital: +$85,000 (increase)
Annual Cash Flows:
Revenue: +$570,000
Costs: -$220,000
Depreciation: Calculate using MACRS depreciation method
Taxable income: Revenue - Costs - Depreciation
Taxes: Calculate using the tax rate of 21%
After-tax cash flow: Taxable income - Taxes
Now let's calculate the annual cash flows using the MACRS depreciation method:
Year 1:
Depreciation: $170,000 (since it's a 7-year class asset)
Taxable income: $570,000 - $220,000 - $24,286 (MACRS depreciation for Year 1)
Taxes: $24,286 * 21%
After-tax cash flow: Taxable income - Taxes
Year 2:
Depreciation: MACRS depreciation for Year 2 (assuming it's different from Year 1)
Taxable income: $570,000 - $220,000 - Depreciation (Year 2)
Taxes: Taxable income * 21%
After-tax cash flow: Taxable income - Taxes
Year 3:
Depreciation: MACRS depreciation for Year 3
Taxable income: $570,000 - $220,000 - Depreciation (Year 3)
Taxes: Taxable income * 21%
After-tax cash flow: Taxable income - Taxes
Finally, we calculate the salvage value (resale value):
Salvage value: $72,000
Now we can sum up all the cash flows:
Year 0: Initial Investment
Year 1: After-tax cash flow
Year 2: After-tax cash flow
Year 3: After-tax cash flow
Year 3: Salvage value
To know more about salvage value, refer here:
https://brainly.com/question/31922161
#SPJ4
question 13 help i’m timed thx u r loved

Answers
Answer:
178.3 L
Explanation:
178,300/1000
CAN SOMEONE PLEASE ANSWER THIS FAST PLEASE!
How many moles of ammonia (NH3) can be produced from the reaction of 4.0 liters of hydrogen at 50.0°C and 1.2atm of pressure with excess nitrogen?
Answers
Answer: Therefore, approximately 0.1247 moles of ammonia can be produced from the given reaction.
Explanation:
To determine the number of moles of ammonia (NH3) produced from the given reaction, we need to use the ideal gas law and stoichiometry.
The balanced chemical equation for the reaction between hydrogen (H2) and nitrogen (N2) to form ammonia (NH3) is:
N2 + 3H2 → 2NH3
From the equation, we can see that three moles of hydrogen react with one mole of nitrogen to produce two moles of ammonia.
First, let's convert the given conditions of hydrogen to the appropriate units for the ideal gas law:
Volume of hydrogen = 4.0 liters
Temperature of hydrogen = 50.0°C = 50.0 + 273.15 = 323.15 K
Pressure of hydrogen = 1.2 atm
Now, let's calculate the number of moles of hydrogen using the ideal gas law equation:
PV = nRT
where:
P = pressure (in atm)
V = volume (in liters)
n = number of moles
R = gas constant (0.0821 L·atm/(mol·K))
T = temperature (in Kelvin)
n(H2) = PV / RT
n(H2) = (1.2 atm) * (4.0 L) / (0.0821 L·atm/(mol·K) * 323.15 K)
≈ 0.187 mol
Since the stoichiometry ratio is 3:2 (H2:NH3), we can conclude that 0.187 moles of hydrogen can produce (0.187/3) * 2 = 0.1247 moles of ammonia.
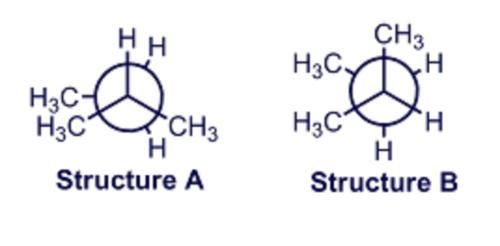
Newman projections are given for two of the conformers of 2-methylbutane, drawn as viewed down the C2-C3 bond. In A the _____ strain is at a maximum due to the eclipsing of bonds. In B this strain is minimized but there is still _____ strain due to the proximity of the bulky CH3 groups that are _____ to each other. Multiple choice question.
Answers
The Newman projection gives the view of the molecule. In molecule A the torsional strain and in B steric strain are present because of the gauche methyl group.
What is a torsional strain?The Newman projection depicts the molecules in the head-on view and uses the letters and the lines to represent the molecule. The torsional strain is because of the molecule's resistance to the twisting.
The steric strain is the Vander wall strain that is due to the forced interaction of the molecules separated by covalent bonds resulting in molecular potential energy.
The diagram for the question is attached below.
Therefore, the correct blanks are torsional, steric, and gauche.
Learn more about torsional strain here:
https://brainly.com/question/27852131
#SPJ1
Balance the following chemical equation (if necessary):
FeCl3(aq) + Na₂S (aq) → Fe₂S3 (S) + NaCl(aq)
Answers
The correct balanced chemical equation is FeCl₃(aq) + Na₂S(aq) → 3Fe₂S₃(s) + 3NaCl(aq)
To achieve a chemical equation's balance:
Fe₂S₃ (s) + NaCl (aq) FeCl₃ (aq) + Na₂2S (aq)
Let's start by balancing the atoms of iron (Fe). The iron atoms are already balanced, since there are two on the reactant side and two on the product side.
Let's now balance the atoms of sodium (Na). Two sodium atoms are present on the reactant side, hence two sodium atoms are required on the product side. By adding a coefficient of 2 in front of NaCl, we may do this:
Let's balance the sulfur (S) atoms lastly.
FeCl3(aq) + Na2S(aq) → 3Fe2S3(s) + 3NaCl(aq)
Learn more about chemical equation, here:
https://brainly.com/question/14457720
#SPJ1
100 POINTS WILL MARK BRAINLIEST PLEASE HELP

Answers
Answer:
B is the answer
Explanation:
Answer:
Accurate but not precise
Explanation:
Give 1 pH value where benzoic acid and dibromobenzene would be in different layers in an extraction. Assume that possible pH values are between 0 and 14.
Answers
The pH value where benzoic acid and dibromobenzene would be in different layers in extraction is 7.
Benzoic acid is a weak acid and has a pKa of 4.2, which means it will exist mostly in the form of the acid (HA) at pH values lower than 4.2 and mostly in the form of the salt (A-) at pH values higher than 4.2.
Dibromobenzene is a neutral compound and its pH does not affect its behavior.
If we take pH = 7 which is higher than the pKa of benzoic acid, benzoic acid will exist mostly in the form of salt (A-) in this pH range and Dibromobenzene will remain neutral.
So, Benzoic acid will be in the aqueous layer and Dibromobenzene will be in the organic layer during extraction.
To learn more about Benzoic acid:
https://brainly.com/question/28299797
#SPJ4
Which of the following reactions would result in decreased entropy?
Q A. CO,(s) → CO2(g)
OB. H₂O(g) → H₂O()
OC. 203(g) → 30₂(g)
D. N₂204(g) → 2NO₂(g)
Answers
The reaction that would result in decreased entropy is : ( A ) CO(s) → CO2(g)
What are Endothermic reactionsReactions that absorb heat from its surroundings are known as endothermic reactions, Endothermic reactions result in a decreased entropy as the temperature of the surrounding becomes lower than the temperature of the container where the reaction occurs.
Examples of endothermic reactions are : melting and evaporation which results in the change of state from solid to liquid or from solid to gaseous state.
Hence we can conclude that The reaction that would result in decreased entropy is : ( A ) CO(s) → CO2(g)
Learn more about endothermic reactions : https://brainly.com/question/10508226
#SPJ1
The greater a habitat's biodiversity, the greater will be the habitat's, sustainability over time with varying conditions sustainability over time with varying conditions consumption of energy in the form of sunlight consumption of energy in the form of sunlight temperature ranges across the seasons temperature ranges across the seasons distance to the nearest water source distance to the nearest water source
Answers
Answer:
The correct answer is: sustainability over time with varying conditions.
Explanation:
A habitat can be defined as the physical and geographical conditions that will positively influence the development of life, in any form.
Therefore, it is correct to say that sustainability over time with favorable conditions will favor the greatness of the habitat.
Sustainability is the ability to preserve a system for the maintenance of future life, so it is ideal for there to be sustainable development across society, so that natural resources are preserved so that biodiversity continues to exist.
In what areas of the country should this material be marked?
Answers
Answer:
Explanation: The goal of every country should be to produce more materials and goods
Learning Task 2: Identify the forms of energy needed. 1. Turning electric fan on 2. Drying clothes 3. Grilling barbecue 4. Moving a bicycle 5. Lighting a candle 6. Music of xylophone 7. missiles and a weapon 8. charging my phone 9. playing a guitar 10. turning on a flashlight
Answers
Turning on a flashlight - Electrical Energy
What is energy conversion, using an example?
From one form to another, energy can be transformed. Examples: Our cars are filled with gasoline (chemical), which, with the aid of electrical energy from a battery, produces mechanical (kinetic) energy. Our TVs are powered by purchased electricity, which is then transformed into light and music.
1. Turning electric fan on - Electrical Energy
2. Drying clothes - Thermal Energy
3. Grilling barbecue - Chemical energy → Thermal energy
4. Moving a bicycle - Chemical energy → Mechanical energy
5. Lighting a candle - Chemical energy → Thermal energy or light energy
6. Music of xylophone - Sound energy → Electrical energy
7. missiles and a weapon - stored electrical energy
8. charging my phone - Electrical energy to chemical energy
9. playing a guitar - Sound energy → Electrical energy
10. turning on a flashlight - Electrical Energy
Learn more about energy conversion
brainly.com/question/11234965
#SPJ1
An unknown amount of Al203 decomposed producing 215 g of solid aluminum. 2Al2O3=4Al+3O2 How many grams of oxygen gas should be produced
Answers
Answer:
191.11 grams of oxygen gas should be produced.
Explanation:
The balanced reaction is:
2 Al₂O₃ → 4 Al + 3 O₂
By stoichiometry of the reaction (that is, the relationship between the amount of reagents and products in a chemical reaction), the following amounts of moles of each compound participate in the reaction:
Al₂O₃: 2 molesAl: 4 molesO₂: 3 molesBeing the molar mass of each compound:
Al₂O₃: 102 g/moleAl: 27 g/moleO₂: 32 g/moleBy reaction stoichiometry, the following mass quantities of each compound participate in the reaction:
Al₂O₃: 2 moles* 102 g/mole= 204 gramsAl: 4 moles* 27 g/mole= 108 gramsO₂: 3 moles* 32 g/mole= 96 gramsThen you can apply the following rule of three: if by stoichiometry 108 grams of aluminum are produced along with 96 grams of oxygen, 215 grams of aluminum are produced along with how much mass of oxygen?
\(mass of oxygen=\frac{215 grams of aluminum*96 grams of oxygen}{108grams of aluminum}\)
mass of oxygen= 191.11 grams
191.11 grams of oxygen gas should be produced.
a hydrogen atom is in the ground state absorbs light with a wavelength of 102.6nm calculate the energy level of the resilting excited state
Answers
Hydrogen atom is in the ground state and absorbs light with a wavelength of 102.6 nm, the energy level of the resulting excited state will be 3.
Ground state (nₐ) = 1
excited state (nₓ) = ?
wavelength = 102.6 nm or 102.6 × 10⁻⁹
Calculate the frequency
v = c / λ
v = 3 × 10⁸ m/s / 102.6 × 10⁻⁹
v = 2.923 × 10¹⁵ s⁻¹
Now calculate the energy
E = nhv
E = 1 × 6.63 × 10⁻³⁴ Js⁻¹ × 2.923 × 10¹⁵ s⁻¹
E = 1.9379 × 10⁻¹⁸
Calculate the excited state (nf) by using the following equation
dE = Rₙ.Z² [ 1 / nₐ² - 1 / nₓ²]
1.9379 × 10⁻¹⁸ ] = 2.18 × 10⁻¹⁸ ] × 1² [1 / 1² - 1 / nₓ²]
1 / nₓ² = 1 - 0.8889
nₓ² = 1 / 0.111
nₓ² = 9
nₓ = 3
You can also learn about energy levels from the following question:
https://brainly.com/question/17396431
#SPJ4
Asexual reproduction produces offspring that are genetically _____________ to the parent.
Answers
Answer:
identical
Explanation:
Asexual reproduction happens through binary fission. Binary fission is when the ‘parent’ creates two new ‘daughter’ cells, which are genetically identical to the parent.
(basically cloning, lol)
what is the molar mass of nickel (ll) chloride(NiCl2)
Answers
Answer:
Explanation:
yt
Answer:
129.5994 g/mol
Explanation:
What is the purpose of adding the aqueous sodium bicarbonate to your reaction mixture?.
Answers
The purpose of adding the aqueous sodium bicarbonate to your reaction mixture is to quench any remaining trace amounts of acid.
An aqueous solution of saturated sodium bicarbonate (NaHCO3) and sodium carbonate (Na2CO3) is simple, and the reason for those washes is to neutralize an organic layer that could include a hint of acidic components. The reason for acidifying an organic base for extraction is to manipulate the solubilities to get transparent layers.
With the alternate solubilities and the usage of pH, the layers can dispose of unwanted additives and place them into the specific layers to be without problems extracted. Which will separate a carboxylic acid compound from the rest of the organic materials, a solution of sodium bicarbonate is added for the duration of extraction. As a vulnerable base, bicarbonate reacts with the carboxylic acid via a neutralization reaction.
Learn more about sodium bicarbonate here:-https://brainly.com/question/20693952
#SPJ4
The molarity of a NaOH solution was determined by titration with KHP. The results of five titrations were 0.1025 M, 0.1087 M, 0.1100 M, 0.1052 M, 0.0997 M. Answer the following questions based on 95% confidence level.
a) Calculate the absolute standard deviation of the concentration of NaOH.
b) Calculate the standard error of the concentration of NaOH.
c) Calculate the confidence interval of the concentration of NaOH. Report your answer with appropriate significant figures
d) If the true concentration of this NaOH solution is 0.1045 M, is the sample mean significantly different from the true concentration?
e) Another student also measured the concentration of the same NaOH solution. The result of the three titrations were 0.1028 M, 0.1012 M, 0.0983 M. Are the mean concentrations from the two students’ result similar to each other?
Answers
a) The absolute standard deviation of the concentration of NaOH is 0.0041 M.
b) The standard error of the concentration of NaOH is 0.0018 M.
c) The confidence interval of the concentration of NaOH is (0.1033 M, 0.1060 M).
d) Yes, the sample mean is significantly different from the true concentration of 0.1045 M.
e) No, the mean concentrations from the two students' results are not similar to each other.
a) To calculate the absolute standard deviation of the concentration of NaOH, we need to find the standard deviation of the given data points. Using the formula for sample standard deviation, we calculate the average deviation of each data point from the mean concentration, then square each deviation, take the average of the squared deviations, and finally, take the square root. The absolute standard deviation is the absolute value of the standard deviation.
b) The standard error of the concentration of NaOH measures the variability of the sample means from different samples. It is calculated by dividing the standard deviation by the square root of the sample size. In this case, the sample size is 5.
c) To calculate the confidence interval of the concentration of NaOH, we need to determine the margin of error using the t-distribution and the sample standard deviation. With a 95% confidence level, we use a t-value corresponding to 4 degrees of freedom (n-1) and multiply it by the standard error. The confidence interval is constructed by subtracting and adding the margin of error to the sample mean concentration.
d) To determine if the sample mean is significantly different from the true concentration, we compare the true concentration to the confidence interval. If the true concentration falls outside the confidence interval, then the sample mean is significantly different from the true concentration.
e) To assess if the mean concentrations from the two students' results are similar to each other, we can calculate the confidence intervals for each student's data. If the confidence intervals overlap or are close to each other, it suggests that the mean concentrations are similar. However, if the confidence intervals do not overlap, it indicates that the mean concentrations are likely different.
Learn more about concentration
brainly.com/question/18247103
#SPJ11
1. Write the formula and give the name for the compounds formed between the
following ions:
a. Cu2+ and Br-
d. Hg2+ and S2-
b. Fe2+ and 02-
e. Sn2+ and F-
c. Pb2+ and Cl-
f. Fe3+ and 02-
HELPPPP MEEEE PLEASEEEE
Answers

Determine the products of the reaction between tin(ii) oxalate and lithium chloride
Answers
The reaction between tin (II) oxalate and lithium chloride is that it forms tin (II) chloride and lithium oxalate, which are the products of the reaction. The balanced chemical equation for the reaction is SnC₂O₄ + 2 LiCl → SnCl₂ + Li₂C₂O4.
Tin (II) oxalate reacts with lithium chloride to form a precipitate of tin (II) chloride and lithium oxalate. The reaction between tin (II) oxalate and lithium chloride is given below.
SnC₂O₄ + 2 LiCl → SnCl₂ + Li₂C₂O4
The balanced chemical equation for the reaction is as follows:
SnC₂O₄ + 2 LiCl → SnCl₂ + Li₂C₂O4 .
SnC₂O₄ is tin (II) oxalate, while LiCl is lithium chloride.
SnCl₂ is tin (II) chloride, while Li₂C₂O4 is lithium oxalate.The products of the reaction between tin (II) oxalate and lithium chloride are tin (II) chloride and lithium oxalate. Tin (II) chloride is a white crystalline powder that is soluble in water, whereas lithium oxalate is a white solid that is insoluble in water.The reaction between tin (II) oxalate and lithium chloride is a double displacement reaction, which is also known as a metathesis reaction. When a double displacement reaction takes place, two compounds exchange their cations and anions, resulting in the formation of two new compounds.
The reaction is a double displacement reaction or metathesis reaction where two compounds exchange their cations and anions to form two new compounds.
To know more about double displacement reaction visit:
brainly.com/question/29740109
#SPJ11
What is the percent of chloride ion in a sample if 2. 500 g of the sample produces 1. 750 g of agcl when treated with excess ag⁺? report your answer to two decimal places.
Answers
The percent of chloride ion in a sample if 2. 500 g of the sample produces 1. 750 g of agcl when treated with excess ag⁺ is 17.2 percent.
What is mass percent?
The ratio of the mass of the solute contained in a solution to the mass of the solution as a whole is known as the mass percent.
Explanation:
Given:
Mass of sample = 2. 500 g
Mass of AgCl = 1. 750 g
The percent of chloride ion in a sample is calculated as,
Molar mass of AgCl = 143.32 g/mol
\(Molar mass of Chlorine atom = 35.45 g/mol\)
Assume that all of the sample's chlorine has precipitated into silver chloride. As a result, the amount of chlorine in silver chloride will match the amount of chlorine in the sample.
First we have to find mass of chlorine,
\(In 143.32 g of silver chloride, mass of chlorine present is 35.45 gSo, in 1.3487 g of silver chloride, mass of chlorine present will be =35.45g/143.32 g * 1.750 g = 0.43 g\)
Therefore, the percent of chloride ion in a sample is
\(Mass of pure chloride compound = 2.500 gMass of chlorine = 0.43 g%Chlorine = 0.43/2.5 *100%Chlorine = 17.2 %\)
Hence, the percent of chloride ion in a sample is \(17.2%\) percent.
To learn more about mass percentage from the given link.
https://brainly.com/question/17463660
#SPJ4
Question 4
Calculate the nlass of 7.1 x 1024 formula units of magnesium hydroxide, Mg(OH),.
Answers
its me again this is how you find the answer. note: i dont have the periodic table to see the exact atomic mass to find the molar mass however this is the answer:
so the actual formula is Mg(OH) with a 2 as a subscript because there are 2 Mg. so with Mg(OH)2 the Molar mass is 58.32g/mol. 58.32 g/mol x 7.1x1024 = 4.1x1026g
Which two ways the nervous and endocrine systems interact?
Answers
Answer:
Along with the nervous system, the endocrine system coordinates the body's functions to maintain homeostasis during rest and exercise. The nervous and endocrine systems also work together to initiate and control movement, and all the physiological processes movement involves.
Explanation:
Which structure is the arrow pointing to?
What is the function of this organelle?

Answers
The structure the arrow is pointing to is the nucleus and it helps store genetic material (DNA) in living cells.
What is an organelle?Organelle is a specialized structure found inside cells that carries out a specific life process. examples are ribosomes, vacuoles, nucleus etc.
The nucleus is the large membrane-enclosed organelle found in eukaryotic cells which contains genetic material.
The nucleus is located at the center of living cells and it functions to store genetic material called DNA. The DNA is transferred to offsprings for inheritance of traits.
According to this question, a microscopic image of a cell is shown. The arrow pointed to the nucleus of the cell.
Learn more about nucleus at: https://brainly.com/question/4031640
#SPJ1
which element has the highest monetary value?
A) gold
B) Silver
C) nickel
D) lead
Answers
Answer:
A. gold has the highest monetary value?
Among the given elements, gold has the highest monetary value. So the correct option is A.
What is gold?
Gold (Au) is a chemical element that belongs to Period 6's Group 11 (Ib) and is a thick, glossy golden valuable metal. Gold has historically been extremely valued due to a number of characteristics. It is typically found in nature in a relatively pure form, is appealing in colour and brightness, resilient to the point of virtual indestructibility, and very flexible.
Due to its perceived worth from the beginning, gold has a history that is unmatched by that of any other metal. Gold is one of the densest metals.
It is a good heat and electrical conductor. It is also the softest, most malleable, and ductile of all the elements; a troy ounce (31.1 grammes) of gold may be hammered into gold leaf, which can be crushed into sheets as thin as 187 square feet (approximately 17 square metres).
Therefore the correct option is A.
Read more about gold, here
https://brainly.com/question/4838993
#SPJ2
Are the following chemical equations reversible or irreversible?
2H2O ←→ H3O+ + OH-
HA + H2O ←→ A- + H3O+
HA + H2O → A- + H3O+
MOH → M+ + OH-
Answers
The first two chemical equations are reversible while the other two are irreversible.
What are chemical equations?Chemical equation is a symbolic representation of a chemical reaction which is written in the form of symbols and chemical formulas.The reactants are present on the left hand side while the products are present on the right hand side.
A plus sign is present between reactants and products if they are more than one in any case and an arrow is present pointing towards the product side which indicates the direction of the reaction .There are coefficients present next to the chemical symbols and formulas .
The first chemical equation was put forth by Jean Beguin in 1615.By making use of chemical equations the direction of reaction ,state of reactants and products can be stated. In the chemical equations even the temperature to be maintained and catalyst can be mentioned.
Learn more about chemical equations,here:
https://brainly.com/question/19626681
#SPJ1
A cup of hot water contains fewer molecules than a tub of hot water. This same cup of hot water will freeze faster than a tub of hot water. The total thermal energy of a system is not only dependent on the state of the material but also on the (resistance, heat, temp, half-life) and the total number of (atoms, currents, compounds, electromagnetic waves) in the system.
Answers
Answer:
The correct options are;
1) Heat
2) Atoms
Explanation:
The heat required to raise a unit mass of a substance by one degree which is the specific heat capacity of the substance, is a quantity characteristic of a given substance and the amount of temperature change experienced by the substance when an amount of heat energy transferred to the substance is a function of the mass of the substance, which is a function of the number of atoms present in the substance.
So also, the total thermal energy that is stored by a substance, that causes a given amount of temperature change, is a function of the heat capacity of the substance.
problem 21.5 which acid derivative in each of these pairs do you think undergoes hydrolysis more quickly? explain.
Answers
The acid derivative in each pair that undergoes hydrolysis more quickly can be determined by looking at the steric hindrance of the carbonyl carbon. Pair A: Ethyl benzoate vs. methyl benzoate and Pair B: N-propyl acetate vs. isopropyl acetate.
The greater the steric hindrance, then it slower the hydrolysis rate. In pair A, ethyl benzoate undergoes hydrolysis more quickly than methyl benzoate because the ethyl group has less steric hindrance than the methyl group. In pair B, isopropyl acetate undergoes hydrolysis more quickly than N-propyl acetate because the branched isopropyl group has less steric hindrance than the straight chain N-propyl group.
Know more about hydrolysis here:
https://brainly.com/question/30468294
#SPJ11
OSTOICHIOMETRY
Using molarity to find solute moles and solution volume
A chemist adds 440.0 mL of a 1.46M barium acetate
added to the flask. Round your answer to 3 significant digits.
mol
be (Ba(C₂H₂O₂),) solution to a reaction flask, Calculate the millimoles of barium acetate the chemist has
X
Calculator
542400
Maribel V
do
Answers
The chemist has 642.4 millimoles of barium acetate in the solution.
To calculate the millimoles of barium acetate (Ba(C₂H₃O₂)₂) in the solution, we can use the formula:
moles = molarity × volume (in liters)
First, let's convert the volume from milliliters (mL) to liters (L):
440.0 mL ÷ 1000 = 0.440 L
Now we can substitute the given values into the formula:
moles = 1.46 M × 0.440 L
moles = 0.6424 mol (rounded to 4 decimal places)
To convert the moles to millimoles, we multiply by 1000:
millimoles = 0.6424 mol × 1000
millimoles = 642.4 mmol (rounded to 3 significant digits)
Therefore, the chemist has 642.4 millimoles of barium acetate in the solution.
It's important to note that the molarity (M) represents the number of moles of solute per liter of solution. By multiplying the molarity by the volume in liters, we can find the number of moles of solute. To convert moles to millimoles, we multiply by 1000. The result represents the millimoles of barium acetate present in the given volume of solution.
For more such questions on barium acetate visit:
https://brainly.com/question/15304103
#SPJ8
According to the first law of thermodynamics, what quantity is conserved?.
Answers
Answer:
energy
Explanation:
The First Law of Thermodynamics (Conservation) states that energy is conserved, it cannot be created nor destroyed.